Gut Health and the Post-Weaning Transition in Piglets
![The relative abundance of the significantly different taxa between Nursing and Weaned piglets at the genus level. The interquartile range is indicated by the outer bounds of the boxes, and the median is indicated by the black midline. The whiskers represent the minimum and maximum values. The [P < 0.001], [P < 0.01] and [P < 0.05] were indicated as [***], [**] and [*], respectively (from Guevarra et al., 2018).](/_next/image/?url=https%3A%2F%2Fimages.engormix.com%2FE_articles%2F0_954_989.gif&w=1080&q=75)
Argü ello H, Estellé J, Leonard FC, Crispie F, Cotter PD, O’Sullivan O, Lynch H, Walia K, Duffy G, Lawlor PG, Gardiner GE. Influence of the intestinal microbiota on colonization resistance to Salmonella and the shedding pattern of naturally exposed pigs. mSystems, v.4, pii: e00021-19. doi: 10.1128/mSystems.00021-19, 2019.
Bischoff SC. ‘Gut health’: a new objective in medicine? BMC Medicine, v.9, 24, 2011.
Castillo M, Martin-Orue SM, Nofrarias M, Manzanilla EG, Gasa J. Changes in caecal microbiota and mucosal morphology of weaned pigs. Veterinary Microbiology, v.124, p.239-247, 2007.
Celi P, Cowieson AJ, Fru-Nij F, Steinert RE, Kluenter A-M, Verlhac V. Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Animal Feed Science and Technology, v.234, p.88-100, 2017.
Celi P, Vivianeb V, Estefania PC, Jeromeb S, Kluenter A-M. Biomarkers of gastrointestinal functionality in animal nutrition and health. Animal Feed Science and Technology, v.250, p.9-31, 2019.
de Lange CFM, Pluske JR, Gong J, Nyachoti CM. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livestock Science, v.134, p.124-134, 2010.
Dou S, Gadonna-Widehem P, Rome V, Hamoudi D, Rhazi L, Lakhal L, Larcher T, Bahi-Jaber N, Pinon-Quintana A, Guyonvarch A, Huerou-Luron ILE, Abdennebi-Najar L. Characterisation of early-life fecal microbiota in susceptible and healthy pigs to post-weaning diarrhoea. PLoS ONE, v.12, n.1, e0169851, 2017.
Gaskins HR. Intestinal bacteria and their influence on swine growth. In: Lewis, A.J.; Southern, L.L. (Eds.). Swine Nutrition, 2nd edition, Florida, USA: CRC Press, p.585-608. 2001.
Guevarra RB, Hong SH, Cho JH, Kim B-R, Shin J, Lee JH, Kang BN, Kim YH, Wattanaphansak S, Isaacson RE, Song M, Kim HB. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. Journal of Animal Science and Biotechnology, v.9, 54, 2018.
Guevarra RB, Lee JH, Lee SH, Seok M-J, Kim DW, Nang BN, Johnson TJ, Isaacson RE, Kim HB. Piglet gut microbial shifts early in life: causes and effects. Journal of Animal Science and Biotechnology, v.10, 1, 2019.
Héctor A, Estellé J, Zaldívar-López S, Jiménez-Marín Á, Carvajal A, López-Bascón MªA, Crispie F, O’Sullivan O, Cotter PD, Priego-Capote F, Morera L, Garrido JJ. Early Salmonella typhimurium infection in pigs disrupts microbiome composition and functionality principally at the ileum mucosa. Scientific Reports, v.8, Article number: 7788, 2018.
Heo JM, Opapeju FO, Pluske JR, Kim JC, Hampson DJ, Nyachoti CM. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. Journal of Animal Physiology and Animal Nutrition, v.97, p.207-237, 2013.
Isaacson R, Kim HB. The intestinal microbiome of the pig. Animal Health Research Reviews, v.13, p.100-109, 2012.
Jayaraman B, Nyachoti CM. Husbandry practices and gut health outcomes in weaned pigs: a review. Animal Nutrition, v.3, p.205-211, 2017.
Kiarie EG, Mills A. Role of feed processing on gut health and function in pigs and poultry: Conundrum of optimal particle size and hydrothermal regimens. Frontiers in Veterinary Science, v.6, 19, 2019.
Kim HB, Borewicz K, White BA, Singer RS, Sreevatsan S, Tu ZJ, Isaacson RE. Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs. Veterinary Microbiology, v.153, p.124-133, 2011.
Kim HB, Isaacson RE. The pig gut microbial diversity: understanding the pig gut microbial ecology through the next generation high throughput sequencing. Veterinary Microbiology, v.177, p.242-251, 2015.
Kim JC, Hansen CF, Mullan BP, Pluske JR. Nutrition and pathology of weaner pigs: Nutritional strategies to support barrier function in the gastrointestinal tract. Animal Feed Science and Technology, v.173, p.3-16, 2012.
Kogut MH, Arsenault RJ. Editorial: gut health: the new paradigm in food animal production. Frontiers in Veterinary Science, v.3, p.71-74, 2016.
Lallès JP. Nutrition and gut health of the young pig around weaning: what news? Archiva Zootechnica, v.11, p.5-15, 2008.
Lallès JP, Bosi P, Smidt H, Stokes CR. Nutritional management of gut health in pigs around weaning. Proceedings of the Nutrition Society, v.66, p.260-268, 2007.
Lallès JP, Boudry G, Favier C, Le Floc'h N, Luron I, Montagne L, Oswld IP, Pie S, Piel C, Seve B. Gut function and dysfunction in young pigs: physiology. Animal Research, v.53, p.301-316, 2004.
Li S, Zheng J, Deng K, Chen L, Zhao XL, Jioang X, Fang Z, Che L Xu S, Feng B, Li J, Lin Y, Wu Y, Han Y, Wu Y. Supplementation with organic acids showing different effects on growth performance, gut morphology, and microbiota of weaned pigs fed with highly or less digestible diets. Journal of Animal Science, v.96, n.8, p.3302-3318, 2018.
Liao SF, Nyachoti M. Using probiotics to improve swine gut health and nutrient utilization. Animal Nutrition, v.3, p.331-343, 2017.
Lindberg JE. Fiber effects in nutrition and gut health in pigs. Journal of Animal Science and Biotechnology, v.5, 15, 2014.
Liu Y, Espinosa CD, Abelilla JJ, Casas GA, Lagos LV, Lee SA, Kwon WB, Mathai JK, Navarro DMVL, Jaworski NW, Stein HH. Non-antibiotic feed additives in diets for pigs: A review. Animal Nutrition, v.4, p.113-125, 2018.
McCracken BA, Spurlock ME, Roos MA, Zuckermann FA, Gaskins HR. Weaning anorexia may contribute to local inflammation in the piglet small intestine. Journal of Nutition, v.129, p.613-619, 1999.
Modina SC, Polito U, Rossi R, Di Giancamillo A. Nutritional regulation of gut barrier integrity in weaning piglets. Animals, 9, n.12, 1045 doi:10.3390/ani9121045, 2019.
Molist F, Gómez de Segura A, Gasa J, Hermes RG, Manzanilla EG, Anguita M, Pérez JF. Effects of dietary fibre on phsycochemical characteristics of digesta, microbial activity and gut maturation in early weaned piglets. Animal Feed Science and Technology, v.149, p.346-353, 2009.
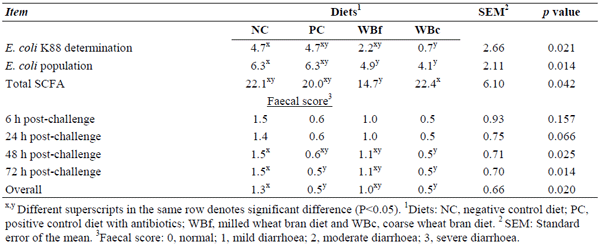
Molist F, Gómez de Segura A, Pérez JF, Bhandari SK, Krause DO, Nyachoti CM. Effect of wheat bran on the health and performance of weaned pigs challenged with Escherichia coli K88+. Livestock Science, v.133, n.1-3, p.214-217, 2010.
Moeser AJ, Pohl CS, Rajput M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Animal Nutrition, v.3, p.313-321, 2017.
Pluske JR. Feed- and feed additives-related aspects of gut health and development in weanling pigs. Journal of Animal Science and Biotechnology, v.4, 1, 2013.
Pluske JR, Hampson DJ, Williams IH. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livestock Production Science, v.51, p.215-36, 1997.
Pluske JR, Turpin DL, Kim J. Gastrointestinal tract (gut) health in the young pig. Animal Nutrition, v.4, p.187-196, 2018.
Pluske JR, Zentek J. Gut nutrition and health in pigs and poultry. In: Hendriks, W.H.; Verstegen, M.W.A.; Babinszky. L. (Eds.). Poultry and Pig Nutrition: Challenges of the 21st Century. Wageningen Academic Publishers: The Netherlands, p.77-103. 2019
Spreeuwenberg MAM, Verdonk JMAJ, Gaskins HR, Verstegen MWA. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. Journal of Nutrition, v.131, p.1520-1527, 2001.
Tugnoli B, Giovagnoni G, Piva A, Grilli E. From acidifiers to intestinal health enhancers: How organic acids can improve growth efficiency of pigs. Animals, v.10, n.1, 134, doi:10.3390/ani10010134, 2020.
Tung CM, Pettigrew JE. Critical Review of Acidifiers. National Pork Board: Des Moines, IA, USA, 2006.
Vanrolleghema W, Tanghea S, Verstringea S, Bruggemana G, Papadopoulos D, Trevisic P, Zentek J, Sarrazine S, Dewulf J. Potential dietary feed additives with antibacterial effects and their impact on performance of weaned piglets: A meta-analysis. The Veterinary Journal, v.249, p.24-32, 2019.
Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, Popova IE, Parikh SJ, Adams LG, Tsolis RM, Stewart VJ, Bäumler AJ. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science, v.339, p.708-711, 2013.
Wijtten PJA, van der Meulen J, Verstegen MWA. Intestinal barrier function and absorption in pigs after weaning: a review. British Journal of Nutrition, v.105, p.967-981, 2011.
Xiong X, Tan B, Song M, Ji P, Kim K, Yin Y, Liu Y. Nutritional intervention for the intestinal development and health of weaned pigs. Frontiers in Veterinary Science, v.6, p.46, 2019.
Zeng MY, Inohara N, Nunez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunology, v.10, p.18-26, 2017.










