Mycotoxins and Mycotoxigenic Fungi in Poultry Feed for Food-Producing Animals
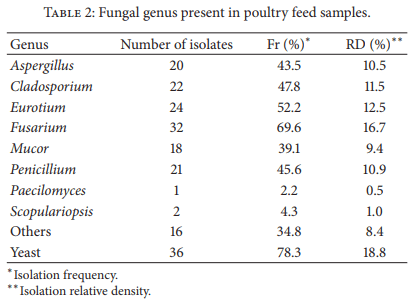
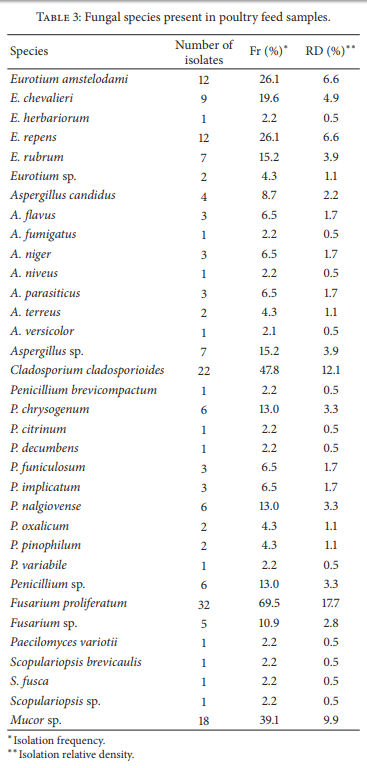
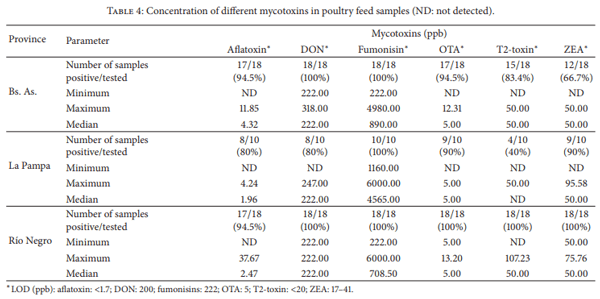
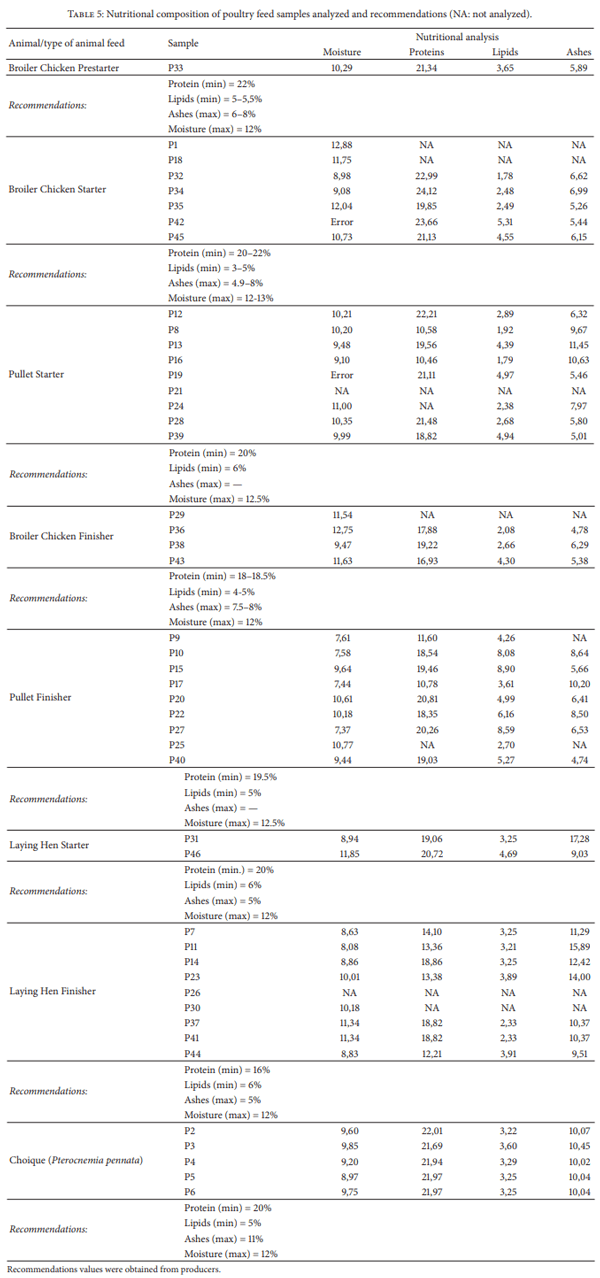
Moulds are capable of reducing the nutritional value of feedstuff as well as elaborating several mycotoxins. Mycotoxin-contaminated feed has adverse effects on animal health and productivity. Also, mycotoxins may be carried over into meat and eggs when poultry are fed with contaminated feed. In a point prevalence study feedstuff used for poultry nutrition in Argentina was analyzed for fungal flora, natural incidence of selected mycotoxins, and nutritional quality. Ten mould genera were recovered, six of them known to be mycotoxigenic. More than 28 species were determined. Fumonisins were detected in all the samples (median 1,750 ppb). Forty-four out of 49 samples (90%) were contaminated with DON (median 222 ppb) and OTA (median 5 ppb). Also, 44 out of 49 samples were contaminated with aflatoxins (median 2.685 ppb), 42 samples (86%) with ZEA (median 50 ppb), and 38 samples (78%) with T2- toxin (median 50 ppb). Ninety percent of the samples had at least one type of nutritional deficiency. This study indicates the need for continuous assessment of the mycological status of animal feed production, in order to feed animals for optimal performance ensuring food safety.
[1] Food and Agriculture Organization of the United Nations (FAO), Statistic Division (FAOSTAT), Trends in the livestock sector, part 3, 2013, http://www.fao.org/docrep/015/i2490e/ i2490e03c.pdf.
[2] R. Cegielska-Radziejewska, K. Stuper, and T. Szablewski, “Microflora and mycotoxin contamination in poultry feed mixtures from western Poland,” Annals of Agricultural and Environmental Medicine, vol. 20, no. 1, pp. 30–35, 2013.
[3] I. C. Okoli, C. U. Nweke, C. G. Okoli, and M. N. Opara, “Assessment of the mycoflora of commercial poultry feeds sold in the humid tropical environment of Imo State, Nigeria,” International Journal of Environmental Science and Technology, vol. 3, no. 1, pp. 9–14, 2006.
[4] J. R. Gillespie and F. B. Flanders, Modern Livestock and Poultry Production, Cengage Learning, Ontario, Canada, 8th edition, 2009.
[5] G. Damerow, The Chicken Encyclopedia: An Illustrated Reference, Storey, North Adams, Mass, USA, 2012.
[6] A. M. Shareef, “Molds and mycotoxins in poultry feeds from farms of potential mycotoxicosis,” Iraqi Journal of Veterinary Sciences, vol. 24, no. 1, pp. 17–25, 2010.
[7] J. Nyamongo and M. Okioma, “The aflatoxin outbreaks in Kenya in 2004 and 2005: a case study,” in Proceedings of the Conference on Reducing Impact of Mycotoxins in Tropical Agriculture with Emphasis on Health and Trade in Africa, p. 3, Accra, Ghana, 2005.
[8] E. M. Binder, L. M. Tan, L. J. Chin, J. Handl, and J. Richard, “Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients,” Animal Feed Science and Technology, vol. 137, no. 3-4, pp. 265–282, 2007.
[9] A. Venancio and R. Paterson, “The challenge of mycotoxins,” in Food Safety—A Practical and Case Study Approach, A. McElhatton and R. J. Marshall, Eds., pp. 24–47, Springer, Berlin, Germany, 2007.
[10] J. Pitt and A. Hocking, Fungi and Food Spoilage, Springer, Berlin, Germany, 3rd edition, 2009.
[11] H. S. Hussein and J. M. Brasel, “Toxicity, metabolism, and impact of mycotoxins on humans and animals,” Toxicology, vol. 167, no. 2, pp. 101–134, 2001.
[12] J. L. Orellano, “Metodos de determinaci ´ on, identificaci ´ on y con- ´ trol de micotoxinas en ingredientes para la nutricion animal,” ´ Engormix, 2007.
[13] A. Gimeno and M. L. Martins, Micotoxinas y Micotoxicosis en Animales y Humanos, Special Nutrients, Miami, Fla , USA, 1st edition, 2007.
[14] T. Mabbett, “Keep feeds free from fungi,” African Farming, pp. 15–16, 2004.
[15] A. R. Khosravi, M. Dakhili, and H. Shokri, “A mycological survey on feed ingredients and mixed animal feeds in Ghom province, Iran,” Pakistan Journal of Nutrition, vol. 7, no. 1, pp. 31–34, 2008.
[16] “Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed,” Official Journal of the European Communities, L 140/10, 2002.
[17] Commission Regulation (EU), “Amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for nitrite, melamine, Ambrosia spp. and carry-over of certain coccidiostats and histomonostats and consolidating Annexes I and II thereto,” Official Journal of the European Union, vol. L159/7, 2011.
[18] “Commission recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding,” Official Journal of the European Union, L229/7, 2006.
[19] A. M. Dalcero, C. E. Magnoli, S. M. Chiacchiera, G. Palacios, and M. Reynoso, “Mycoflora and incidence of aflatoxin B1, zearalenone and deoxynivalenol in poultry feeds in Argentina,” Mycopathologia, vol. 137, no. 3, pp. 179–184, 1997.
[20] A. M. Dalcero, C. E. Magnoli, M. Luna et al., “Mycoflora and naturally occurring mycotoxins in poultry feeds in Argentina,” Mycopathologia, vol. 141, no. 1, pp. 37–43, 1998.
[21] C. E. Magnoli, A. M. Dalcero, S. M. Chiacchiera, R. Miazzo, and M. A. Saenz, “Enumeration and identification of ´ Aspergillus group and Penicillium species in poultry feeds from Argentina,” Mycopathologia, vol. 142, no. 1, pp. 27–32, 1998.
[22] C. E. Magnoli, M. A. Saenz, S. M. Chiacchiera, and A. M. Dal- ´ cero, “Natural occurrence of Fusarium species and fumonisinproduction by toxigenic strains isolated from poultry feeds in Argentina,” Mycopathologia, vol. 145, no. 1, pp. 35–41, 1999.
[23] C. E. Magnoli, S. M. Chiacchiera, R. Miazzo et al., “The mycoflora and toxicity of feedstuffs from a production plant in Cordoba, Argentina,” ´ Mycotoxin Research, vol. 18, pp. 7–22, 2002.
[24] M. del Pilar Monge, C. E. Magnoli, and S. M. Chiacchiera, “Survey of Aspergillus and Fusarium species and their mycotoxins in raw materials and poultry feeds from Cordoba, Argentina,” ´ Mycotoxin Research, vol. 28, no. 2, pp. 111–122, 2012.
[25] M. P. Monge, A. M. Dalcero, C. E. Magnoli, and S. M. Chiacchiera, “Natural co-occurrence of fungi and mycotoxins in poultry feeds from Entre R´ios,” Food Additives and Contaminants B, vol. 6, pp. 168–174, 2013.
[26] R. A. Samson, E. S. Hoekstra, and J. C. Frisvad, Introduction to Food and Airborne Fungi, Centraalbureau voor Schimmelcultures, Utrech, The Netherlands, 7th edition, 2004.
[27] P. Nelson, T. Toussoun, and W. Marasas, Fusarium Species: An Illustrated Manual for Identification, Pennsylvania State University Press, University Park, Pa, USA, 1983.
[28] E. Simmons, Alternaria: An Identification Manual, American Society of Microbiology, Washington, DC, USA, 1st edition, 2009.
[29] H. H. Gonzalez, S. L. Resnik, R. T. Boca, and W. F. Marasas, “Mycoflora of Argentinian corn harvested in the main production area in 1990,” Mycopathologia, vol. 130, no. 1, pp. 29–36, 1995.
[30] A. M. Pacin, H. H. L. Gonzalez, M. Etcheverry, S. L. Resnik, ´ L. Vivas, and S. Espin, “Fungi associated with food and feed commodities from Ecuador,” Mycopathologia, vol. 156, no. 2, pp. 87–92, 2003.
[31] M. K. Saleemi, M. Z. Khan, A. Khan, and I. Javed, “Mycoflora of poultry feeds and mycotoxins producing potential of Aspergillus species,” Pakistan Journal of Botany, vol. 42, no. 1, pp. 427–434, 2010.
[32] M. V. Greco, A. G. Pardo, V. Ludemann, P. E. Martino, and G. N. Pose, “Mycoflora and natural incidence of selected mycotoxins in rabbit and chinchilla feeds,” The Scientific World Journal, vol. 2012, Article ID 956056, 6 pages, 2012. [33] AOAC, Official Methods of Analysis, Association of Official Analytical Chemists, Gaithersburg, Md, USA, 16th edition, 1997.
[34] M. D. Castillo, H. H. L. Gonzalez, E. J. Mart ´ ´inez, A. M. Pacin, and S. L. Resnik, “Mycoflora and potential for mycotoxin production of freshly harvested black bean from the Argentinean main production area,” Mycopathologia, vol. 158, no. 1, pp. 107– 112, 2004.
[35] A. Gimeno, “Los Hongos y las Micotoxinas en la Alimentacion´ Animal; Conceptos, Problemas, Control y Recomendaciones,” 2002, http://www.engormix.com/MA-micotoxinas/articulos/ criterios-calidad-micologica-t353/p0.htm.
[36] M. L. G. Pereyra, V. A. Alonso, R. Sager et al., “Fungi and selected mycotoxins from pre- and postfermented corn silage,” Journal of Applied Microbiology, vol. 104, no. 4, pp. 1034–1041, 2008.
[37] M. L. Gonzalez Pereyra, C. M. Pereyra, M. L. Ram ´ ´irez, C. A. R. Rosa, A. M. Dalcero, and L. R. Cavaglieri, “Determination of mycobiota and mycotoxins in pig feed in central Argentina,” Letters in Applied Microbiology, vol. 46, no. 5, pp. 555–561, 2008.
[38] G. N. Pose, Estudio de la biodiversidad de especies de Fusarium contaminantes de trigo y ma´iz en la provincia de Santa Fe [Ph.D. thesis], Universidad Nacional del Litoral, 2002.
[39] G. R. Oliveira, J. M. Ribeiro, M. E. Fraga et al., “Mycobiota in poultry feeds and natural occurrence of aflatoxins, fumonisins and zearalenone in the Rio de Janeiro State, Brazil,” Mycopathologia, vol. 162, no. 5, pp. 355–362, 2006.
[40] M. Jaramillo, Aditividad , Sinergismo y Antagonismo entre Micotoxinas y sus Efectos en Pollos de Engorde, 2006, http://www.engormix.com/MA-avicultura/sanidad/articulos/ aditividad-sinergismo-antagonismo-entre-t972/p0.htm.
[41] A. Di Constanzo and M. Murphy, “St rategies for Feeding Mycotoxin and Mold Contaminated Grains to Cattle,” 2012, http://www.extension.umn.edu/agriculture/beef/components/ docs/strategies for feeding mycotoxin and mold contaminated grain.pdf.







.jpg&w=3840&q=75)