The probiotic Enterococcus faecium modifies the intestinal morphometric parameters in weaning piglets
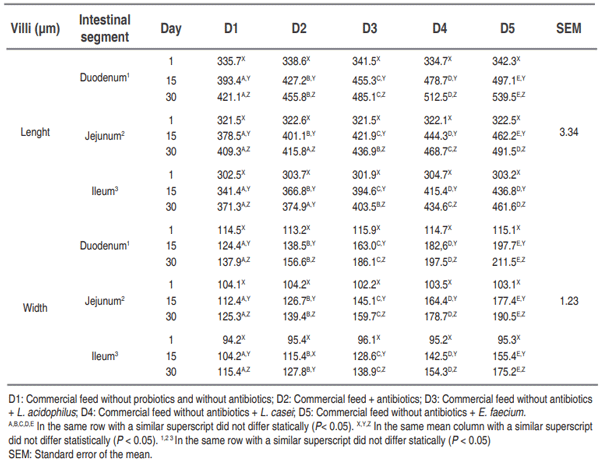
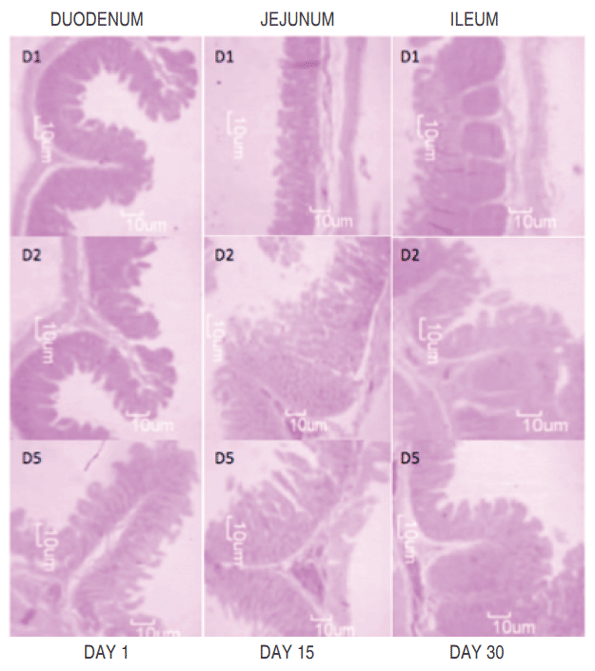
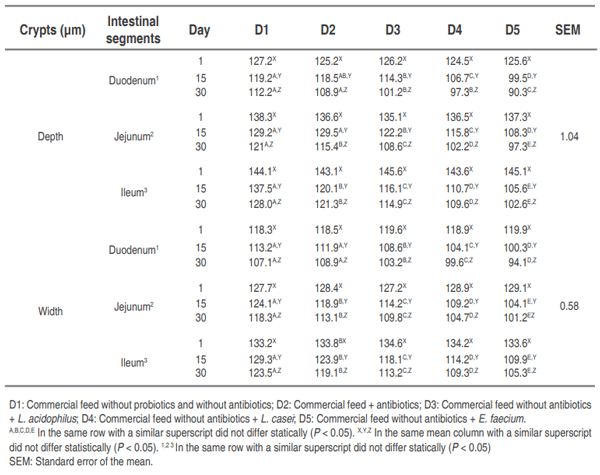
Global trends for animal production have seen a decrease in the use of antimicrobial compounds in feed, generating the need to implement new nutritional strategies that stimulate growth and promote intestinal health. This study aimed to determine whether the addition of E. faecium in drinking water improves intestinal morphometric parameters in post- weaning pigs compared with the probiotics strains L. acidophilus and L. casei on days 1 (21 days of age), 15 and 30 postweaning. The small intestine was completely removed to evaluate the morphometric parameters (length and width of villi and crypts) in the different intestinal segments (duodenum, jejunum, and ileum). They were fed for 30 days with two diets: commercial diet with or without antibiotics. The different probiotics, L. acidophillus, L. casei and E. faecium, were administered in the drinking water of the animals that consumed the commercial diet without antibiotics. A randomized block design in split-plot arrangement was used. There was a significant increase (P<0.01) in the width and length of villi, and a decrease (P<0.01) in the values obtained for the width and depth of crypts in the animals that consumed E .faecium, as compared to those that consumed the diet with addition of antibiotics. The use of probiotics, especially E. faecium, is a nutritional treatment strategy when antimicrobial compound are used, improving the intestinal morphometric parameters and, at the same time, the digestive and productive parameters of the animals. Work is in progress to investigate the effects of probiotic supplementation on the mofication of gut microbiota of post-weaning piglets.
Key words: Weaning, Small intestine, Piglets, Probiotics, Villi.

Bednorz, C., S. Guenther, K. Oelgeschläger, B. Kinnemann, R. Pieper, S. Hartmann, K. Tedin, T. Semmler, K. Neumann, P. Schierack, A. Bethe and L.H. Wieler. 2013. Feeding the probiotic Enterococcus faecium strain NCIMB 10415 to piglets specifically reduces the number of Escherichia coli pathotypes that adhere to the gut mucosa. Applied Environmental Microbiology 79(24): 7896- 904. doi: 10.1128/AEM.03138-13.
Cabrera, R.A., J.L. Usry, C. Arrellano, E.T. Nogueira, M. Kutschenko, A.J. Moeser and J. Odle. 2013. Effects of creep feeding and supplemental glutamine or glutamine plus glutamate (Aminogut) on pre- and post-weaning growth performance and intestinal health of piglets. Journal of Animal Sciences and Biotechnology 4(1): 29. doi: 10.1186/2049-1891-4-29
Campbell, J.M., J.D. Crenshaw and J. Polo. 2013. The biological stress of early weaned piglets. Journal of Animal Sciences and Biotechnology 4(1): 19. doi: 10.1186/2049-1891-4-19
CIOMS. Council For International Organization Of Medical Sciences And The International Council For Laboratory Animal Science. 2012. International guiding principles for biomedical research involving animals. Disponible en: http://grants.nih.gov/ grants/olaw/Guiding_Principles_2012.pd
Ciro, J.A., A. López and J.E. Parra. 2013. Expresión molecular de la vilina en yeyuno de lechones posdestete que consumieron LPS de E. coli. Revista CES Medicina Veterinaria y Zootecnia 8(2): 32-41. doi: 10.15446/rfmvz.v61n2.44677
DiRienzo, D.B. 2014. Effect of probiotics on biomarkers of cardiovascular disease: implications for heart-healthy diets. Nutrition Reviews 72(1): 18-29.doi: 10.1111/nure.12084
FAO/OMS. Organización de las Naciones Unidas para la Agricultura y la Alimentación. 2006. Probióticos en los alimentos, propiedades nutricionales y directrices para la evaluación. Disponible en: ftp://ftp.fao.org/docrep/fao/009/a0512s/a0512s00.pdf
Farzan, A., J. Kircanski, J. DeLay, G. Soltes, J.G. Songer, R. Friendship and J.F. Prescott. 2013. An investigation into the association between cpb2-encoding Clostridium perfringens type A and diarrhea in neonatal piglets. Canadian Journal of Veterinary Research 77(1): 45-53.
Flesch, A.G., A.K. Poziomyck and D. de C. Damin. 2014. The therapeutic use of symbiotics. ABCD: Arquivos Brasileiros de Cirurgia Digestiva 27(3): 206-209. doi: 10.1590/s0102-67202014000300012
Fre S, M. Huyghe, P. Mourikis, S. Robine, D. Louvard and S. Artavanis-Tsakonas. 2005. Notch signals control the fate of immature progenitor cells in the intestine. Nature 435: 964–968.
Ghosh, T.S., S.S. Gupta, G.B. Nair and S.S. Mande. 2013. In silico analysis of antibiotic resistance genes in the gut microflora of individuals from diverse geographies and age-groups. PLoS One 8(12): e83823. doi: 10.1371/journal.pone.0083823
Gómez IAS, D. Vergara and F. Argote. 2008. Efecto de la dieta y edad del destete sobre la fisiología digestiva del lechón. Revista Biotecnología en el Sector Agropecuario y Agronindustrial 6: 32-41.
Jacobi, K.S., J.A. Moeser, T.A. Blikslager, M.J. Rhoads, A.B. Corl, J.R. Harrell and J. Odle. 2013. Acute effects of rotavirus and malnutrition on intestinal barrier function in neonatal piglets. World Journal of Gastroenterology 19(31): 5094–5102. doi: 10.3748/wjg. v19.i31.5094
Kang, P., D. Toms, Y. Yin, Q. Cheung, J. Gong, K. De Lange and J. Li. 2010. Epidermal growth factor-expressing Lactococcus lactis enhances intestinal development of early-weaned pigs. The Journal of Nutrition. 140(4): 806-811. doi: 10.3945/jn.109.114173
Klingspor S, H. Martens, D. Caushi, S. Twardziok, J.R. Aschenbach and U. Lodemann. 2013. Characterization of the effects of Enterococcus faecium on intestinal epithelial transport properties in piglets. Journal of Animal Science; 91(4): 1707-1718. doi: 10.2527/ jas.2012-5648
Lallès J.P., S. Konstantinov and H.J. Rothkötter. 2004. Bases physio- logiques, microbiologiques et immunitaires des troubles digestifs du sevrage chez le porcelet: don nées récentes dans le contexte de la suppression des antibiotiques additifs alimentaires. Journees Recherche Porcine. 2004; 36: 139-150.
Marion, J., M. Biernat, F. Thomas, G. Savary, Y. Le Breton, R. Zabielskil, I. Le Huërou-Lurona and J. Le Dividich. 2002. Small intestine growth and morphometry in piglets weaned at 7 days of age. Effects of level of energy intake. Reproduction and Nutrition Development 42: 339–354. doi: 10.1051/rnd:2002030
Mitsuoka, T. 2014. Development of functional foods. Bioscience of Microbiota, Food and Health 33(3): 117-28.
Müller A, M. Oertli and I.C. Arnold. 2011. Pylori exploits and manipulates innate and adaptive immune cell signaling pathways to establish persistent infection. Cell Communication and Signaling. 9(1): 25. doi: 10.1186/1478-811X-9-25.
NRC. 2012. National Research Council. The Nutrient Requirements of Swine. 8th rev. ed. Washington, DC, USA: National Academy Press.
Parra, S.J., T.J. Agudelo, M.C. Ramírez, B. Rodríguez and H.A. López. 2011. Lipopolysaccharide (LPS) from E. coli has detrimental effects on the intestinal morphology of weaned pigs. Revista Colombiana de Ciencias Pecuarias 24: 598-608.
Reis, S.T.C., C.M.J. Guerrero, B.A. Aguilera and L.G. Mariscal. 2005. Efecto de diferentes cereales sobre la morfología intestinal de lechones recién destetados. Revista Mexicana de Ciencias Pecuarias 43: 309-321.
Reis, S.T.C., L.G. Mariscal, B.A. Aguilera and J.G.H. Cervantes. 2007. Digestibilidad de la proteína y energía en dietas para lechones complementadas con tres diferentes tipos de suero de leche deshidratado. Veterinaria México 38: 141-151.
SAS®. SAS/STAT User’s Guide. Institute Inc. Statistical Analysis Systems Institute. Version 9.1th Ed. Cary, NC: SAS Institute Inc. 2007.
Schokker, D., J. Zhang, L.L. Zhang, S.A. Vastenhouw, H.G. Heilig, H. Smidt, J.M. Rebel and M.A. Smits. 2014. Early-life environmental variation affects intestinal microbiota and immune development in new-born piglets. PLoS One. 9(6): e100040. doi: 10.1371/journal. pone.0100040
Segalés, J y M. Domingo. 2003. La necropsia en el ganado porcino, diagnóstico anatomopatológico y toma de muestras. Boehringer Ingelheim S.A, Madrid, España. 128 p.
Siggers, R.H., J. Siggers, M. Boye, T. Thymann, L. Molbak, T. Leser, B.B. Jensen and P.T. Sangild. 2008. Early administration of probiotics alters bacterial colonization and limits diet-induced gut dysfunction and severity of necrotizing enterocolitis in preterm pigs. The Journal of Nutrition 138(8): 1437-44. doi: 10.1152/ ajpgi.00414.2007
Songisepp, E., P. Hütt, M. Rätsep, E. Shkut, S. Kõljalg, K. Truusalu, J. Stsepetova, I. Smidt, H. Kolk, M. Zagura and M. Mikelsaar. 2012. Safety of a probiotic cheese containing Lactobacillus plantarum Tensia according to: A variety of health indices in different age groups. Journal of Dairy Science 95(10): 5495-509. doi: 10.3168/ jds.2011-4756
Suo C, Y. Yin, X. Wang, X. Lou, D. Song, X. Wang and Q. Gu. 2012. Effects of Lactobacillus plantarum ZJ316 on pig growth and pork quality. BMC Veterinary Research. 25; 8: 89-101. doi: 10.1186/1746-6148-8-89.
Suzuki K, H. Fukui, T. Kayahara, M. Sawada, H. Seno, H. Hiai, R. Kageyama, H. Okano and T. Chiba. 2005. Hes1-deficient mice show precocious differentiation of Paneth cells in the small intestine. Biochemical and Biophysical Research Communications 328: 348–352.
Vente-Spreeuwenberg M.A.M., A.C. Verdonk, H.R. Gaskins, and M.W.A. Verstegen. 2001. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. Journal of Nutrition 131: 1520-1527.
Wang Z, W. Chai, M. Burwinkel, S. Twardziok,, P. Wrede, C. Palissa, B. Esch and M.F. Schmidt. 2013. Inhibitory influence of Enterococcus faecium on the propagation of swine influenza: A virus in vitro. PLoS One8(1): e53043. doi: 10.1371/journal.pone.0053043.
Zeyner, A and E. Boldt. 2006. Effects of a probiotic Enterococcus faecium strain supplemented from birth to weaning on diarrhoea patterns and performance of piglets. Journal of Animal Physiology and Animal Nutrition 90: 25–31.
Zhenfeng Z, O. Deyuan, P. Xiangshu, W.K. Sung, L. Yanhong and W. Junjun. 2008. Dietary arginine supplementation affects microvascular development in the small intestine of early-weaned pigs. Journal of Nutrition 138: 1304-1309.









.jpg&w=3840&q=75)