Milk Microbiology: Improving Microbiological Services for Dairy Farms
Published: December 7, 2016
By: Pamela L. Ruegg, DVM, MPVM, University of WI, Dept. of Dairy Science, Madison WI 53705
Introduction
In spite of considerable progress in improvement of milk quality, mastitis continues to be the most frequent and costly disease of dairy cows, however few veterinarians are actively involved in mastitis control programs. On most farms, detection, diagnosis and administration of treatments for clinical mastitis are the responsibility of farm personnel and veterinarians are often consulted only when a case threatens the life of the cow. Several studies have indicated that many dairy veterinarians are only marginally involved in mastitis control programs. Only 24% of dairy farmers (n = 180) enrolled in a milk quality program in Wisconsin indicated that they used their herd veterinarian to plan milk quality programs (Rodrigues et al., 2005). In a companion survey, most dairy veterinarians (n = 42) interested in participating in a mastitis control program indicated that they spent <10% of their professional time actively working to improve milk quality (Rodrigues and Ruegg. 2004). There are ample economic and societal reasons for veterinarians to increase their involvement in mastitis control programs. The occurrence of mastitis reduces milk production, increases the amount of milk discarded and increases premature culling and production costs (Fetrow, 2000). Additionally, both clinical and subclinical mastitis have been demonstrated to reduce reproductive efficiency (Barker et al., 1998, Schrick, 2001). Involvement in implementation of a milk quality plan as part of the production medicine program can result in increased demand for veterinary services and improved economic performance for the dairy farm. The purpose of this paper is to provide an introduction of how veterinarians can use milk culture data to improve mastitis control programs.
Use of Milk Culturing on Dairy Farms
Mastitis is a bacterial disease and control is based on understanding the etiology of the infection. As dairy farms increase in size, an increasingly diverse group of pathogens have been associated with the occurrence of mastitis. Clinical signs may be suggestive of some pathogens but it is impossible to diagnose the etiology based on the appearance of the milk, gland or animal so almost all mastitis experts recommend the use of milk culturing to direct mastitis control programs. In spite of this advice relatively few farmers extensively use a culturing program (Hoe and Ruegg, 2005; Table 1). This data indicates a clear association between herd size and adoption of culturing.
Table 1. Frequency of submitting samples for culturing by Wisconsin Dairy Farmers
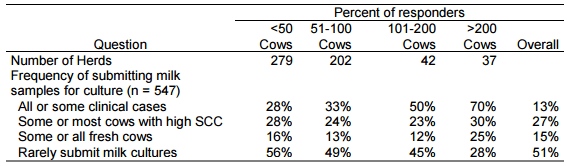
Diagnostic tests (such as milk cultures) are most useful when results are closely linked to management decisions. Most traditional technologies, such as submission of milk samples for culture or require sending milk samples to a remote laboratory. These methods are removed from the farm and have been criticized as too slow for on-farm decision making. Anecdotal data suggests that farmers don’t adopt the use of milk culturing because they don’t know how to use the results or recognize the economic value of the decisions that are made as a result of the test. Results of milk culturing can be very useful to identify mastitis pathogens and make management decision about treatment, culling, segregation and disease control programs.
Practical Aspects of Collecting and Using Milk Culture Data
Milk samples may be individually collected from affected quarters (quarter milk samples) or combined from all four glands into a single vial (composite milk samples). Quarter milk samples are more sensitive in detection of bacteria from subclinical infections as compared to composite samples although the sensitivity of composite samples increases with the number of infected glands per cow. Mastitis occurs when teats are exposed to pathogenic bacteria that are able to overcome teat end defenses and stimulate a detectable immune response. Mastitis is therefore almost always caused by a single type of bacteria and laboratory protocols indicate that the recovery of >2 types of bacteria from a single milk sample is defined as contamination during collection (NMC, 1999). The use of composite milk samples, almost always results in a greater proportion of contaminated samples and this mode of sample collection should be reserved for screening for subclinical infections caused by organisms such as Streptococcus agalactiae and Mycoplasma bovis. Pooling of 5-10 aseptically collected milk samples is another strategy that is used in some herds to further reduce sampling costs. When either of these strategies is used it is important to recognize the potential for false negative outcomes is increased. In all instances, sensitivities increase as the number of infected mammary gland quarters increase. When using pooled or composite samples, veterinarians should routinely review cow histories to identify cows with increased SCC that are typical of cows with subclinical infections.
Cows are generally more cooperative before milking and more likely to stand still to allow collection of a clean sample. To maximize the possibility of recovering bacteria from the sample, milk should be collected after the teats have been prepared for milking (application of pre-milking disinfectant and drying) but before the units are attached. Milk samples need to be cooled immediately and should be cultured on farm as soon as possible or submitted to the laboratory within 24 hours of collection. If samples cannot be processed within 24 hours, they should be frozen until transported to the lab. Freezing for periods of <2 weeks has minimal effects on recovery of most mastitis causing bacteria but can reduce recovery of Mycoplasma spp.
When specific bacterial diagnoses are needed, it is important that the milk samples are submitted to a laboratory that has experience working with mastitis bacteria. Standardized laboratory methods for identification of mastitis pathogens have been well defined by the National Mastitis Council (1999) and should be the basis for most identifications. While the methods used in most mastitis laboratories are simple, evaluation of culture results requires experienced personnel that have worked with milk samples. In most laboratories the objective is to rapidly identify likely mastitis pathogens and complex methods are not generally needed. In general, technicians inoculate media that contains nutrients required for bacterial growth and the inoculated media is placed in an incubator that contains the appropriate atmosphere and temperature to encourage growth of the target organisms. In most mastitis laboratories, 10 to 100 μL of each milk sample are inoculated onto a portion of a blood agar plate. The inoculum volume determines the lower limit of detection. For example, one colony observed on a plate inoculated with 0.01-mL (10-μL) is equivalent to approximately 100 CFU/mL of milk while one colony observed using a 0.1-mL (100-μL) inoculum is equivalent to approximately 10 CFU/mL. The use of larger inoculum volumes increases sensitivity but also increases the possibility of contamination.
After inoculation, agar plates are incubated at 37°C and observed for growth at 24 and 48 hours. While there is no absolute definition of IMI, the presence of at least 100-300 cfu/mL is usually required to define an infection. Identification of bacteria is made based on phenotypic characteristics of the colonies and the result of additional laboratory tests. Staphylococcus aureus is usually differentiated from other staphylococci based on a positive coagulase reaction and other typical phenotypic characteristics, such as hemolysis. Streptococci are usually identified using the Christie-Atkins-Munch-Petersen (CAMP) test and esculin reactions. When milk samples originate from cases of clinical mastitis, MacConkey agar is usually also inoculated to facilitate the rapid identification of Gram-negative, lactose-fermenting organisms (coliforms). Additional biochemical tests are required for final identification at the species level. Identification of Mycoplasma spp. requires the use of media containing specific nutrients not found in general medias and incubation in a CO2 enhanced environment.
Use of on Farm Culturing to Make Mastitis Treatment Decision.
As dairy herds have increased in size and developed specialized labor forces, mastitis treatment plans that include the use of on-farm culturing have been developed (Hess, et al., 2003). In general, upon diagnosis of a clinical case of mastitis, the cow is examined and assigned a severity score and a milk sample is obtained. Recording of standardized severity scores can help veterinarians better define the pattern of clinical mastitis on individual farms. One severity scoring system uses a 3-point scale that combines the appearance of milk with the progression to additional clinical signs (1 (mild mastitis) = abnormal milk only; 2 (moderate mastitis) = abnormal milk & abnormal udder; 3(severe) = systemic symptoms) (Pinzon-Sanchez et al., 2011). This system is practical, simply recorded and can be an important way to assess detection intensity. In most herds, the distribution of clinical mastitis by severity is approximately 40-50% mild cases, 40-50% moderate cases and 5-15% of the cases scored as severe (Oliveira et al, submitted). If the proportion of severe cases is excessive it is a signal that detection intensity and case definition should be investigated.
If the case is scored as a grade 1 or 2 (mild to moderate mastitis), then the use of OFC program to direct treatment may be considered. In some OFC programs, no antibiotic treatment is given until results of the OFC are known (generally24 hours). Alternatively, a farmer may begin treatment (after collection of the milk sample) but plan to readjust (change the duration of end the therapy) therapy 24 hours later, after the initial culture results are known. Laboratory methods used for OFC are not usually the same as those that are used in professional diagnostic laboratories and are often performed by farm personnel who may have some technical expertise but lack formal training in microbiological methods. On most farms, OFC methods are based on the use of laboratory shortcuts and have a goal of rapidly reaching a presumed diagnosis to guide treatment. The most basic method of OFC is the use of biplates, triplates or quadplates that contain selective medias. Growth on a selective media is used to differentiate cases as caused by Gram-positive or Gram-negative bacteria, culture-negative cases or in some instances specific pathogens. After 24 hours of incubation, culture plates are observed and the treatment protocol is specified based on the culture outcome. Typical agars that are used include: MacConkey agar (selective for growth of Gram-negative bacteria); TKT agar (selective for growth of Streptococci) and Factor, Baird-Parker or KLMB medias (selective for growth and differentiation of some staphylococci). Studies have indicated that using selective medias in OFC systems is about 80% accurate in differentiating Gram positive and Gram negative pathogens as compared to diagnostic laboratories. The use of OFC to make more specific pathogen diagnoses is not as accurate and requires additional training of personnel.
Most smaller herds do not have sufficient cases of mastitis to develop the expertise needed for OFC and one alternative is to offer in-veterinary clinic culturing (IVCC). In these instances, farmers usually collect a milk sample and immediately initiate treatment but they may stop or modify treatment duration or the drug after receiving a preliminary microbiological diagnosis from their veterinarian within 24 hours. Development and oversight of a culture program (either OFC or IVCC) is an ideal way for veterinarians to increase involvement in mastitis control programs. Some veterinary practices provide increase their involvement by offering complete technical support for OFC systems. The use of veterinary technicians to supervise these programs may also increase veterinary involvement and oversight of mastitis treatments. When OFC is used, veterinary technicians can visit farms to restock supplies, train farm personnel and provide oversight and quality control.
Evaluating and Using Results.
The interpretation of results of milk cultures is dependent on the organism, sampling method and laboratory procedures. On modern US dairy herds, when samples are properly collected and processed, approximately 25-30% will be culture negative, 30-35% will be Gram-negative, 25% will be Gram-positive and about 10% will be a variety of other opportunistic organisms. The proportion of culture negative samples that are diagnosed using OFC should be monitored as an indicator of the quality of the sampling and laboratory procedures.
Increasing the use of diagnostic methodologies is an excellent way for veterinary practitioners to improve the efficiency and efficacy of mastitis treatments. The use of OFC to direct treatment of clinical mastitis gives farmers the opportunity to make better treatment decisions and reduce costs associated with milk discard and treatment of microbiologically negative cases (Lago et al., 2011a,b). A positively controlled clinical trial evaluating OFC demonstrated that there were no significant differences in either long-term or short-term outcomes for cases of mastitis that received treatment based on results of OFC as compared to cases treated immediately without regard to diagnosis. (Lago et al., 2011a,b). In this study, antimicrobials were not administered to cases that were culture negative or Gram negative thus the use of intramammary antimicrobials was reduced by approximately 50% as compared to cases which were treated without prior diagnosis. Even more dramatic results were obtained in a study conducted on a large Michigan dairy. In this study, OFC was used to direct treatment of cows with mild to moderate cases of clinical mastitis. Cows that were infected with Gram-positive organism received antimicrobial treatment while cows with no organism isolated or Gram-negative organisms were excluded from therapy. This reduced the number of treated cows by 80% 152 (Hess et al, 2003). It is apparent that selective treatment of mild clinical mastitis based on OFC or IVCC does not affect long-term outcomes, but does decrease antibiotic use. If farms do not routinely culture milk from cows with clinical mastitis, it is still important to determine the most frequent clinical mastitis pathogens in the herd to make appropriate treatment and prevention recommendations. It is particularly important to determine if Mycoplasma species or opportunistic pathogens that are nonresponsive to antibiotic therapy are contributing to clinical mastitis.
Conclusion
Development of an appropriate mastitis control strategy and selection of antibiotics for treatment of mastitis must be based on a presumptive diagnosis of the type of pathogen that is most likely responsible for the infection. While some farms are using on-farm culture systems to help define treatments, other farms may benefit if veterinarians provide similar services in a limited in-veterinary clinic laboratory. Even when OFC or IVCC cannot be provided, it is still extremely important to have at least some culture data routinely collected so that treatment protocols can be appropriately defined. When contagious mastitis is part of the problem, milk samples from fresh cows should be cultured to identify cows that are candidates for post-calving treatments, segregation or other interventions. The most cost-effective treatment protocols are those that are targeted for specific pathogens and provision of these services is an excellent way to increase veterinary involvement in mastitis control on dairy farms.
Presented at Central Veterinary Conference, Kansas City, MO, August 2013.
References
Barker, A. R., F. N. Schrick, M. J. Lewis, H. H. Dowlen, S. P. Oliver. 1998. Influence of clinical mastitis during early lactation on reproductive performance of Jersey cows. J. Dairy Sci. 81:1285-1290.
Erskine, R. J., S. A. Wagner, and F.J. DeGraves. 2003. Mastitis therapy and pharmacology. in Veterinary Clinics of North America. Food Animal Practice. 19(1):109-138.
Fetrow, J. 2000. Mastitis: An economic consideration. in Proceeding 39th Annual Conference National Mastitis Council, Atlanta GA, Feb 13-16, pp 3-47
Hess, J., L. Neuder, and P. Sears. 2003. Rethinking clinical mastitis therapy. . In Proceedings of the 42nd Annual Meeting National Mastitis Council. Jan 26-29, Fort Worth TX, pp372-373.
Hoe, F.G.H., and P. L. Ruegg. 2005. Relationship between antimicrobial susceptibility of clinical mastitis pathogens and treatment outcomes. J Am. Vet. Med. Assoc. 227:1461-1468.
Lago A, Godden SM, Bey R, et al., The selective treatment of clinical mastitis based on on-farm culture results I: Effects on antibiotic use, milk withholding time and short-term clinical and bacteriological outcomes. 2011a; J Dairy Sci 84:4441-4456.
Lago A, Godden SM, Bey R, et al., The selective treatment of clinical mastitis based on on-farm culture results II: Effects on lactation performance including, clinical mastitis recurrence, somatic cell count, milk production and cow survival. 2011b; J Dairy Science 94:4457-4467
National Mastitis Council. 1999. Laboratory handbook on bovine mastitis. 1999. NMC, Madison WI.
Oliveira, L., C. Hulland, and P. L. Ruegg. Characterization of clinical mastitis occurring in cows on 51 large dairy herds in Wisconsin. In review J Dairy Science
Pinzón-Sánchez C, Ruegg PL. Risk factors associated with short-term post-treatment outcomes of clinical mastitis. J Dairy Sci 2011;94:3397-3410.
Rodrigues, A.C.O., D. Z. Caraviello, and P. L. Ruegg. 2005. Management of Wisconsin Dairy herds enrolled in Milk Quality Teams. J. Dairy Sci. 88:2660-2651.
Schrick, F. N., M. E. Hockett, A. M. Saxton, M. J. Lewis, H. H. Dowlen, S. P. Oliver. 2001. Influence of subclinical mastitis during early lactation on reproductive parameters. J. Dairy Sci. 84: 1407-1412.
Related topics:
Authors:
University of Wisconsin - USA
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.





