Classification of metals
Biological chemistry and absorption of inorganic and organic trace metals
Trace elements may be generally defined as those which occur or are required at relatively low concentrations in living tissues. Classically, they have been subdivided into two categories: those which have been established as essential for life or health, and those for which proof of essentiality does not (yet) exist. Although the essentiality of some trace elements is still a matter for debate, it is widely accepted that the trace elements now considered to be essential or beneficial to mammalian and avian species are: arsenic (As), boron (B), chromium (Cr), cobalt (Co), copper (Cu), fluorine (F), iodine (I), iron (Fe), manganese (Mn), molybdenum (Mo), nickel (Ni), selenium (Se), silicon (Si), vanadium (V) and zinc (Zn). Trace element supplementation of animal diets has traditionally been achieved through the use of inorganic salts such as copper (II) sulphate.
However, since a host of intrinsic and extrinsic factors are known to affect the bioavailability of dietary inorganic trace elements, continuous efforts have been made over the years to improve their utilization by humans and animals. It is now well established that metal chelates of, for example, Cu2+, Zn2+ and Mn2+ with amino acids and peptides can enhance the bioavailability of these trace elements, thereby leading to improvements in parameters such as growth, reproduction and general health status when they are otherwise unavailable in sufficient amounts to meet animal needs. Today there are many such forms of metal complexes available in the marketplace for use in animal nutrition; and these have (perhaps unfortunately) been generically entitled ‘organic trace minerals’ by virtue of the fact that the trace elements in question are complexed or otherwise associated with organic molecules.
In view of the increasing use of these products by the animal feed industry, it is of interest to briefly review the essential biochemical functions of at least some of the essential trace elements to establish why it is so desirable to increase their biological availability and to explore some of the possible mechanisms by which the aforementioned organic trace elements can achieve this.
The role of trace metals in biological systems
Even a cursory inspection of the available literature reveals that the involvement of trace metals in key biochemical processes is immense and quite beyond the scope of this particular text (for review see Fenton, 1995). A broad classification of metallobiomolecues (Figure 1) shows the association of trace elements with easily recognisable key enzymes, transport proteins and other systems which are essential for life. A summary of trace mineral biological functions is in Table 1.
Table 1. Nutritional aspects of zinc, copper, iron and manganese.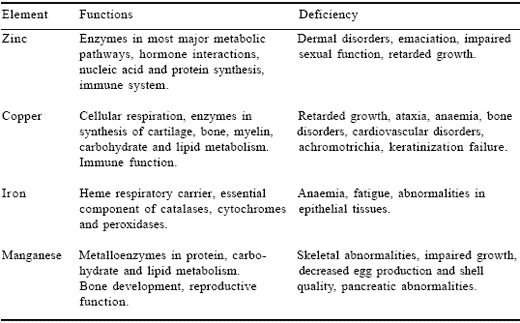
Iron has a vital role in many biochemical reactions. It plays an active part in oxidation/reduction reactions and electron transport associated with cellular respiration. It is found in complexes bound to proteins such as haem, in enzymes such as microsomal cytochromes, catalase, etc., and in non-haem compounds such as transferrin, ferritin and flavin iron-enzymes. Haemoglobin occurs in erythrocytes while transferrin is found in plasma. The latter is the principal carrier of iron in blood. In general terms, iron is essential to cellular and whole body energy and protein metabolism and is vital for good health and the prevention of anaemia (Kaim and Schwederski, 1993).
Copper is very important for animals as it is an essential component of physiologically important metalloenzymes such as cytochrome oxidase, superoxide dismutase, lysyl oxidase, dopamine hydroxylase and tyrosinase.
Overall, this metal is involved in cellular respiration, cardiac function, bone formation, connective tissue development, keratinisation and pigmentation of tissue, as well as myelination of the spinal cord (McDowell, 1992). Copper has a direct effect on iron metabolism and thus indirectly affects haemoglobin biosynthesis. The first sign of copper deficiency experimentally observed was anaemia in rats. In those early studies haemoglobin regeneration was used to estimate the bioavailability of various copper compounds (Schultze et al., 1934; 1936) and in later years to assess copperamino acid and copper-peptide complexes (Kirchgessner and Grassman, 1970). The overall growth-promoting effects of copper are well established and are amply reviewed elsewhere (Underwood, 1977; McDowell, 1992).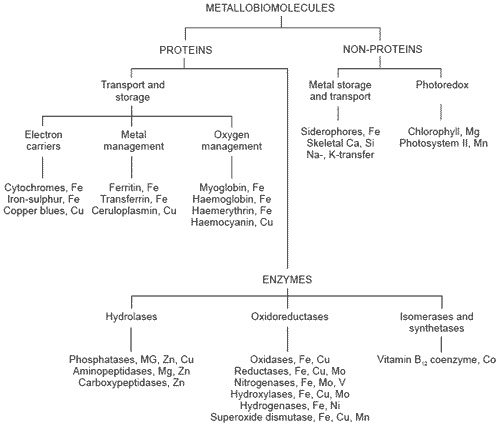
Figure 1. A classification of some metallobiomolecules.
Manganese, like other essential trace elements, can function both as an enzyme activator and as a constituent of metalloenzymes. Manganese– containing enzymes include arginase, pyruvate carboxylase and Mn-superoxide dismutase. Although the number of Mn-metalloenzymes is limited, a large number of enzymes can be activated by manganese. These include hydrolases, kinases, decarboxylases and transferases. Manganese is a vital element for correct bone growth, carbohydrate and lipid metabolism, immune and nervous system function and reproduction. Indeed, effects on reproduction were among the first signs of Mn deficiency to be noted.
Zinc is the second most abundant trace element in mammals and is required as a component of over 300 enzymes in different species of all phyla, (Vallee and Falchuk, 1993). These include carbonic anhydrase, alcohol dehydrogenase and alkaline phosphatase. In its association with enzymes, zinc plays an active catalytic role, generally as a strong Lewis acid, or acts in a regulatory or structural role. Biologically, it is a trace element of immense importance having significant effects on the production and secretion of steroid and peptide hormones which, of course, may offer an explanation for some of the well documented effects of zinc deficiency such as growth retardation, impaired reproductive development/function, aberrant water and cation balance and parakeratosis.
Apart from its well established structural role in key metalloenzymes, zinc performs unique structural functions at a nucleic acid level. The transcription of DNA to RNA and the ultimate translation of the latter to protein is initiated by regulatory proteins called transcription factors. Certain transcription factors such as steroid hormone receptors possess strikingly similar structural organisation. In particular, they contain a distinctive structural motif; the zinc finger, which allows the transcription factor to bind to the corresponding recognition sequence of the target gene thereby regulating its expression. The highly conserved amino acid sequence for zinc fingers contains nine cysteine residues. For each zinc finger formed, zinc is enfolded in tetrahedral co-ordination via donor atoms from cysteine and histidine (Evans and Hollenberg, 1988). Computer-assisted searches for related sequences have revealed potential zinc finger sequences in several classes of proteins involved in nucleic acid recognition. Such findings underline the essentiality of zinc in many critical biological processes.
Increasing the bioavailability of dietary trace metals
From the foregoing very brief overview of the broad functions of some essential trace elements, it is obvious why efforts have been made to increase their biological availability through the use of metal complexes or chelates. The various categories of organic trace metals used in agricultural practice have been defined by the Association of American Feed Control Officials (AAFCO, 1998). These are shown in Table 2. Chelation refers to a specific type of complex formation between a metal ion and a ligand. A ligand in this case may be defined as a molecule containing an atom which has a lone pair of electrons. Metal ions in complexes are bonded to the ligand through donor atoms such as oxygen, nitrogen or sulphur. Chelation occurs where such ligands bond to a metal ion via two or more donor atoms to form a complex containing one or more heterocyclic rings containing the metal atom. Amino acids are examples of ‘bidentate’ ligands, which bond to metal ions via an oxygen of the carboxylic acid group and the nitrogen of the amino group (Hynes and Kelly, 1995). Obviously, not all metal complexes are chelates.
In view of the space which would be required to review all of the available literature on the categories of organic trace minerals listed in Table 2, considerations will be restricted to the most commonly used organic trace mineral supplements, namely; proteinates/chelates of zinc, copper, iron and manganese.
Table 2. AAFCO definitions for organic mineral complexes.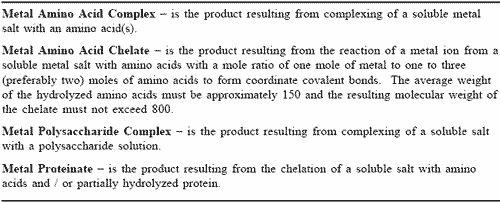
The term ‘bioavailability’ has been the source of considerable debate and is not explained similarly by all investigators. It has been defined as the proportion of the total mineral/nutrient in a food utilized for normal body functions (Fairweather – Tait, 1992). Others have defined bioavailability as the efficiency with which a natural or manufactured source of an element delivers the element to storage or supplies it to metabolically active tissue or to a protein (Wapnir, 1998). It is also considered to reflect the efficiency with which consumed nutrients are absorbed from the alimentary tract and are thus available for storage or use (Forbes and Erdman, 1983; Bender, 1989). Similarly, Ammerman et al. (1995) defined bioavailability as the degree to which an ingested nutrient in a particular source is absorbed in a form that can be utilized in metabolism by the animal. Bioavailability encompasses the sum of impacts that may reduce or promote the metabolic utilization of a nutrient (Schumann et al., 1997). If agreement can be reached, therefore, it is that bioavailability involves both the absorption and the ultimate metabolic utilization of nutrients within the cell.
Physiochemical factors affect nutrient uptake from the intestinal lumen and the incorporation of nutrients into complex biochemical pathways within the cellular environment. On balance, however, impaired intracellular utilization of absorbed inorganic nutrients may not be the major component when considering their overall bioavailability. The aforementioned physiochemical factors that reduce uptake of mineral nutrients from the intestine are the predominant influence on this parameter (Dreosti, 1993). Some of these factors relate obviously to the chemical form of the element or to the presence of other inorganic ions that compete for the same uptake mechanism. Others are caused by the interaction of mineral nutrients with carrier molecules that enhance absorption via specific mucosal receptors or with other organic molecules that reduce it (Table 3). Examples of the latter include phytate, certain sugars, fibre sources and polyphenols. The overall complexity of these interactions is further compounded by the fact that host-related variables also influence mineral availability. These include age, sex, stage of growth, pregnancy, lactation, nutritional status, disease, gastrointestinal secretions and microflora as well as gastrointestinal transit time(Johnson, 1989; Fairweather-Tait, 1996).
Table 3. Examples of dietary factors that increase or decrease absorption, excretion or utilization of certain trace elements.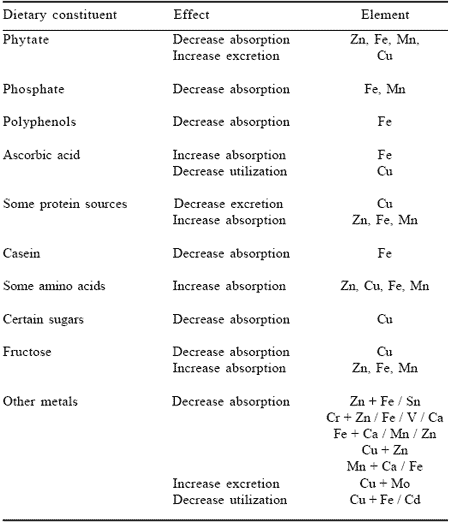
In view of all of these factors, it is not surprising that much remains to be learned in terms of specific uptake mechanisms and utilization of natural and manufactured sources of trace elements, despite the intensive research attention which the area has received over the years.
BIOAVAILABILITY OF TRACE METAL CHELATES AND PROTEINATES
Several investigators have looked at the bioavailability of metal chelates and proteinates relative to inorganic sources. It will be recalled (Table 2) that proteinates are defined as trace metals chelated to amino acids and/or partially hydrolyzed protein. Studies in poultry have revealed notable differences in the bioavailability of zinc from different sources. For example, it has been reported that the bioavailability of zinc from zinc methionine was 206% relative to that of zinc sulphate (Wedekind et al., 1992). Other indicators of the improved bioavailability of organic zinc versus inorganic sources in poultry include studies which document significantly positive effects on gonad development in breeding cocks receiving zinc proteinate versus zinc sulphate (Suchy et al., 1998). In addition, recent studies have demonstrated that the replacement of inorganic zinc and manganese sources with zinc and manganese proteinate improved eggshell quality (Miles, 1998).
Indeed, many studies on the relative bioavailability of manganese have been conducted with poultry for which there is a critical supplemental need for the element. Early studies with poultry, in which growth or leg deformities were measured, were not sufficiently sensitive to detect differences in bioavailability among supplemental sources. However, over the last two decades, tissue deposition of the element has been used to estimate manganese bioavailability. Such studies have revealed that the most available sources of manganese are manganese-methionine and manganese proteinate (Henry, 1995).
In ruminants, copper proteinate has been reported to be more bioavailable than cupric sulphate in studies involving beef cattle (Hemken et al, 1993). Further studies by the same group in dairy cows demonstrated an increased hepatic iron content in copper proteinate versus cupric sulphate-supplemented animals, suggesting that copper proteinate did not interfere with iron uptake and storage as might be expected with inorganic copper sources. In addition, it was found that copper proteinate–supplemented cows had lower plasma ceruloplasmin activity than cows fed cupric sulphate even though plasma copper was essentially the same for both groups (Du et al., 1995). These data suggested that copper proteinate was absorbed via a different mechanism (perhaps even in an intact form) to cupric sulphate and was transported in the blood without binding to ceruloplasmin. In relation to plasma copper levels arising from the use of different copper sources, it is interesting to note that the bioavailability of copper proteinate relative to cupric sulphate has been calculated to be either 147% or 112% depending on whether liver copper or plasma copper is used as the response criterion (Baker and Ammerman, 1995; Kincaid et al.; 1986). Such findings are in agreement with recommendations that liver copper levels, not plasma copper levels, are a better indicator of copper status and relative bioavailability between sources (Lee et al., 1988; Xin et al., 1991).
A number of other studies have compared proteinates with inorganic forms of Cu, Zn, Fe and Mn. Unfortunately, many of these studies have used a combination of organic supplements, which makes it difficult to ascribe specific effects to individual metals. Nevertheless, these studies have demonstrated benefits versus inorganic supplements in reducing somatic cell counts and the incidence of clinical mastitis (Boland et al., 1996). Similarly, Spain (1993) noted fewer (P<0.05) new mammary infections in cows fed zinc proteinate compared to cows fed zinc oxide.
In pigs, a major goal has been to improve the iron status of the newborn piglet through the use of more bioavailable iron sources. For example, iron chelated to amino acids has been reported to lead to increased transfer of iron across the placenta and into the foetus (Ashmead and Graff, 1982). When provided at 200 ppm in the gestation diet, greater quantities of Fe were incorporated into the foetuses resulting in significantly reduced mortality and heavier piglets at birth and weaning (Ashmead, 1996). When employed over eight parities, there were fewer stillborn piglets and more piglets weaned in each parity as well as a shorter interval between weaning and oestrus. Similar effects have been reported for iron proteinate incorporated in a normal late gestation/lactation diet fed from seven days prefarrowing and throughout a 26 day lactation. This led to improved feed intake of the sows and increased weaning weight of the piglets (Close, 1999). In other recent studies, piglets from sows whose diets were supplemented with iron proteinate 21 days pre-farrowing had a higher erythrocyte count (P<0.05) and haemoglobin level (P<0.01) than piglets from sows receiving inorganic iron sources. In addition, liver iron levels were notably higher in piglets from the proteinate supplemented group (Egeli et al., 1998).
Mineral uptake in the gastrointestinal tract
It is evident from the literature that clear differences in bioavailability exist between sources of the same trace metal, with metal proteinates and chelates proving superior to inorganic sources in many cases. As discussed earlier, if it can be accepted that uptake of metals from the intestine is the predominant factor influencing their bioavailability, then differences in uptake mechanisms or in the general presentation of the metal in organic versus inorganic form in the intestinal lumen are likely explanations for the differences noted. The theory that metal amino acid chelates and proteinates utilise peptide and amino acid uptake mechanisms rather than normal metal ion uptake mechanisms in the intestine has become widely accepted (Ashmead et al., 1985; Ashmead, 1993). The basic concept of this theory is that the metal in question is ‘protected’ within the complex in a chemically inert form due to the co-ordinate covalent and ionic bonding by the amino acid ligands. Consequently, the metal is not susceptible to the range of physicochemical factors which can adversely affect the efficient uptake of ‘unprotected’ metal ions. Furthermore, it is believed that the metal chelate is absorbed intact through the intestinal mucosa, effectively pulling the metal along with it.
The metal chelate traverses the mucosal cell membrane, the mucosal cell and basement membrane surviving, still intact, into the plasma. It is very tempting to accept this suggested uptake mechanism, even in part, because it explains numerous practical observations from studies on the relative bioavailability of organic versus inorganic trace metal sources. The reductions noted in negative interactions between elements such as copper, iron and zinc when organic supplements are used agree well with this theory (Hemken et al., 1996). For example, if copper in a proteinated form utilises a peptide uptake mechanism, it is rendered unavailable to compete with iron for regular metal ion uptake mechanisms. This readily explains results such as those reported by Du et al. (1995) which were discussed earlier.
Chemically inert metal complexes would also be protected from negative interactions with dietary constituents such as phytate, which binds cations making them unavailable for absorption (Fairweather-Tait, 1996). Furthermore, transmucosal passage of intact peptides and the existence of peptide carriers in brush-border membranes which utilise a proton-gradient transport mechanism is now firmly established (Gardner, 1998). Indeed, there is good evidence that amino acid absorption in the form of peptides is as important or perhaps even more important than absorption of free amino acids in both ruminants and monogastric animals (Webb et al., 1992; 1993; Rerat and Nunes, 1988). Metals using either amino acid or peptide uptake mechanisms would therefore be expected to be absorbed and circulated to target tissues very efficiently.
Nevertheless, while considerable circumstantial evidence exists to support such a metal uptake mechanism, direct experimental evidence has failed to identify it. In many respects, this is not surprising given the difficulties of establishing even semi-quantitative models in vitro to study such mechanisms. Simulated studies of mineral availability to animals and humans involve closed systems, which theoretically can be described by a set of equations describing the interactions of the chemical components in that system. In vivo systems are not closed, and nutrients neither enter nor leave at a steady state. Because of the non-steady state nature of mammalian digestion and the fact that major parameters such as luminal pH and rates of passage are in a state of flux following meal ingestion makes the design of meaningful in vitro systems with which to study mineral uptake mechanisms very difficult. Our current state of knowledge is that there is no conclusive evidence to support the uptake of trace metal chelates or proteinates in intact form through the utilization of amino acid or peptide uptake mechanisms. Indeed, a number of publications suggest that complexes such as zinc methionine are not, in fact, absorbed as intact entities (Hill et al., 1987; Hempe and Cousins, 1989; House, 1999).
Although there is strong evidence to suggest that selenium in the form of selenomethionine enters the enterocyte via the electrogenic Na+-dependent neutral amino acid transport system in a manner kinetically indistinguishable from that of methionine (Wolffram et al., 1989; Vendeland et al., 1994), selenomethionine cannot be directly compared to the metal amino acid chelates and proteinates which are under consideration here. In selenomethionine, selenium replaces sulphur as an intrinsic part of the amino acid. It would be inaccurate therefore to extrapolate findings on uptake mechanisms for selenomethionine to complexes such as zinc methionine, other metal amino acid chelates or proteinates.
Any consideration of uptake mechanisms for metal complexes cannot ignore the possible effects of gastrointestinal pH on the stability or dissociation of such complexes. This topic has been reviewed in a concise and informative manner by Hynes and Kelly (1995), who demonstrated the species distribution of a number of copper-glycine and zinc-glycine complexes acids and peptides have stability constants of such magnitude as to allow the metal ions to be transferred to the recipient biological system, they presented several important findings in relation to the popularly held theory that metals in complexes are absorbed in a chemically inert form as part of the intact complex. Their findings were as follows:-
1. The distribution of metal species present at given concentrations of metal and amino acids depends on the pH of the solution.
2. Complexed forms (chelates) of dispositive metal ions are not necessarily neutral.
3. Different metal ions have different stability constants and thus the percentage of a metal present as a particular species will depend not only on the pH of the solution but also on the stability constant of the complex.
Taking all such factors into account, it cannot be assumed that metal amino acid chelates and proteinates owe their superior metal bioavailability to uptake mechanisms which allow them to be absorbed as amino acids or peptides in disguise. In the event that such mechanisms do not exist, it is of obvious interest to investigate alternative explanations for the improvements in bioavailability noted for these complexes.
ALTERNATIVE MECHANISMS
Although much effort has been directed towards the identification of metal ion and chelate transport mechanisms, the initial handling of metal ions in the intestinal lumen has received relatively little attention. In this respect, ingested metals may be considered in two categories: those soluble throughout the potential pH range of the gastrointestinal lumen such as Na, Mg and Ca and those susceptible to hydroxy-polymerisation such as Cu, Fe, Mn and Zn. The latter group, termed ‘hydrolytic metals’ also includes potentially toxic metals such as Al. They are acid-soluble but as the pH is raised in the absence of soluble binding ligands, they readily hydroxy-polymerise to form insoluble precipitates.
Recent work strongly suggests that normal metal ion uptake requires both endogenous soluble ligands and mucosally associated ligands to be present in the gut. The former prevent hydroxy-polymerisation of cations such as copper, iron and zinc, while the latter allows some specificity of absorption between toxic and essential metals (Whitehead et al., 1996). The predominant mucosally-associated ligand is thought to be the large glycoprotein mucin, which was once termed ‘gastroferrin’. Mucin is secreted throughout the gastrointestingal tract and provides both the mucosally-adherent gelatinous layer and a soluble luminal form. The role of mucin in metal binding has been well documented (Crowther and Marriott, 1984; Conrad et al., 1991). The affinity of gastrointestinal mucin for metals follows the pattern M3+ > M2+ > M+ and binding may occur at more than one binding site on mucin since the molecule contains sulphated groups (sulphated mucins) and carboxylate groups (sialomucins).
In addition, it has been reported that Zn has two pH-dependent binding sites on mucin (Powell et al., 1999a) and this could be true for other metals. In such a complex binding system it is obvious that there may be competition between different metals for mucin and between mucin and different ligands for metals. This would obviously affect how mucin promotes the availability of dietary metals to the mucosally-adherent mucus layer for the next phase of absorption. In the mucosally-adherent mucus layer, metal binding to mucus also follows the pattern M3+ > M2+ > M+ and for metal absorption the pattern is M+ > M2+ > M3+ (Whitehead et al., 1996). Thus the mucus layer acts as a filter in regulating metal uptake and the strength of binding to and rate of passage across the mucosally-adherent mucus layer could be important in determining the overall absorption of a metal (Powell et al., 1999b). For example, Al3+ will be tightly bound by mucus, has kinetically slow rates of ligand exchange and is therefore unlikely to pass quickly through this layer. In summary, metal absorption from the gastrointestinal lumen for Cu, Fe, Mn and Zn depends on a number of factors including the extent of prevention of luminal hydroxy- polymerisation, the rates of metal ligand exchange and the rate of passage across the mucosally-adherent mucus layer.
Superior absorption/bioavailability of metal amino acid chelates and proteinates versus inorganic sources could therefore be explained in a number of ways. For example, even if a complex is dissociatively labile in the gastrointestinal tract it may still, at least transiently, interfere with metal hydrolysis to allow a more effective presentation of the metal to mucin. As explained by Hynes and Kelly (1995), the extent of this ‘protection’ will be dictated by factors such as pH and the stability constant of the complex itself. Alternatively, as suggested by Powell et al. (1999b), if a ligand is present in significant quantities and is sufficiently strong to compete with mucus for binding of the metal, it may facilitate the rate of passage of the metal through this barrier. Such a mechanism would readily explain many of the reported observations on mineral bioavailability, including the variation in absorption of different elements, the effects of different ligands on mineral uptake and the competition for absorption between different metals.
Conclusions
Significant evidence indicates that amino acid and peptide complexes of metals such as Fe, Zn, Cu and Mn are more bioavailable than inorganic salts. This has led to the belief that these metal complexes are absorbed intact in a chemically inert form using peptide or amino acid transport mechanisms. While such a mechanism for metal uptake may exist, it has not been directly demonstrated to date. Furthermore, conditions in the gastrointestinal tract make it likely that some dissociation of such complexes will occur. Nevertheless, it is not necessary for amino acid chelates or proteinates to be present in an intact, electrically neutral form in order to be absorbed efficiently. The superior uptake of metals contained in such complexes may be explained by an alternative mechanism. For example, their constituent ligands may slow the rate of hydroxy-polymerisation of the metal and allow its effective donation to higher molecular weight binding ligands such as mucin, thereby maintaining them soluble and available to the mucosa for effective absorption.
References
Authors: RONAN POWER and KARINA HORGANAAFCO. 1998. Official Publication of the Association of American Feed Control Officials Incorporated (Paul. M. Bachman, ed). page 237-238.
Ammerman, C.B., D.B. Baker and A.J. Lewis. 1995. Bioavailability of nutrients for animals. Academic Press, New York. p. 441.
Ashmead, H.D. 1993. Comparative intestinal absorption and subsequent metabolism of metal amino acid chelates and inorganic metal salts. In: The Roles of Amino Acid Chelates in Animal Nutrition. (H.D. Ashmead, ed). Noyes Publishers, New Jersey, pp 306-319.
Ashmead, H.D. and D.J. Graff. 1982. Placental transfer of chelated iron. Proceedings of the International Pig Veterinary Society Congress, Mexico. p. 207.
Ashmead, H.D., D.J. Graff and H.H. Ashmead. 1985. Intestinal Absorption of Metal Ions and Chelates. Charles C. Thomas, Springfield, Illinois. Ashmead, H.D. 1996. Nutrition of the high-producing first parity sow. Proceedings of the XVII ANAPORC Symposium, Santiago de Compostela, Spain.
Baker, D.H. and C.B. Ammerman. 1995. Copper bioavailability. In: Bioavailability of Nutrients for Animals. (C.B. Ammerman, D.H. Baker and A.J. Lewis, eds). Academic Press, San Diego, pp. 127-156.
Bender, A.E. 1989. Nutritional significance of bioavailability. In: Nutrient Availability: Chemical and Biological Aspects. (D.A.T. Southgate, I.T Johnson and G.R. Fenwick, eds). Special Publication No. 72, Royal Society of Chemistry, Cambridge, pp 3–9.
Boland, M.P., G. O’Donnell and D. O’Callaghan. 1996. The contribution of mineral proteinates to production and reproduction in dairy cattle. In: Biotechnology in the Feed Industry. (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, Nottingham, United Kingdom, pp. 95–103.
Close, B. 1999. Organic minerals for pigs: an update. In: Biotechnology in the Feed Industry. (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, Nottingham, UK, pp. 51–60.
Conrad, M.E., J.N. Umbreit and E.G. Moore. 1991. A role for mucin in the absorption of inorganic iron and other metal cations: A study in rats. Gastroenterology 100:129–136.
Crowther, R.S. and C. Marriott. 1984. Counter-ion binding to mucus glycoproteins. J. Pharm. Pharmacol 36:21–26.
Dreosti, I.E. 1993. Recommended dietary intakes of iron, zinc and other inorganic nutrients and their chemical form and identity. Nutrition 9:542– 545.
Du, Z., R.W. Hemken and T.W. Clark. 1995. Copper proteinate may be absorbed in chelated form by lactating holstein cows. In: Biotechnology in the Feed Industry. (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, Nottingham, UK, pp. 315–319.
Egeli, A.K., T. Framstad and D. Greeningen. 1998. The effect of peroral administration of amino acid-chelated iron to pregnant sows in preventing sow and piglet anaemia. Acta Vet. Scand. 39:77–87.
Evans, R.M. and S. M. Hollenberg. 1988. Zinc fingers: gilt by association. Cell 52:1–3.
Fairweather-Tait, S.J. 1992. Bioavailability of trace elements. Food Chem. 43:213-217.
Fairweather-Tait, S.J. 1996. Bioavailability of dietary minerals. Biochem. Soc. Trans. 24:775–780.
Fenton, D.E. 1995. Biocoordination Chemistry. Oxford University Press, Oxford, UK, pp. 1-92.
Forbes, R.M. and J.W. Erdman. 1983. Bioavailability of trace mineral elements. Ann. Rev. Nutr. 3:213-231.
Gardner, M.L.G. 1998. Transmucosal passage of intact peptides. In: Peptides in Mammalian Protein Metabolism. (G.K. Grimble and F.R.C. Blackwell, eds). Portland Press, London, pp. 11-29.
Hemken, R.W., T.W. Clark and Z. Du. 1993. Copper: Its role in animal nutrition. In: Biotechnology in the Feed Industry. (T.P. Lyons, ed). Alltech Technical Publications, Nicholasville, Kentucky, USA, pp. 35–39.
Hemken, R.W., Z. Du and W. Shi. 1996. Use of proteinates to reduce competition from other trace minerals. In: Biotechnology in the Feed Industry. (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, Nottingham, UK, pp. 91–94.
Hempe, J.M. and R.J. Cousins. 1989. Effect of EDTA and zinc-methionine complex on zinc absorption by rat intestine. J. Nutr. 119:1179–1187.
Henry, P.R. 1995 Manganese bioavailability. In: Bioavailability of Nutrients for Animals (C.B. Ammerman, D.H. Baker and A.J. Lewis, eds). Academic Press, New York, pp. 239–256.
Hill, D.A., E.R. Peo and A.J. Lewis. 1987. Influence of picolinic acid on the uptake of 65Zn-amino acid complexes by the everfed rat gut. J. Anim. Sci. 65:173–178.
House, W.A. 1999. Trace element bioavailability as exemplified by iron and zinc. Field Crops Research 60:115-141.
Hynes, M.J. and M.P. Kelly. 1995. Metal ions, chelates and proteinates. In: Biotechnology in the Feed Industry (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, Nottingham, UK pp. 233–248.
Johnson, P.E. 1989. What can in vitro methods tell us about mineral availability? Biological Trace Element Research 19:3–10.
Kaim, W. and B. Schwederski. 1993. Uptake, transport and storage of an essential element as exemplified by iron. In: Bioinorganic Chemistry: Inorganic elements in the Chemistry of Life. (W. Kaim and B. Schwederski, eds). John Wiley and Sons, London, pp. 150–171.
Kincaid, R.L., R.M. Blauwiekel and J.D. Conrath. 1986. Supplementation of copper as copper sulfate or copper proteinate for growing calves fed forages containing molybdenum. J. Dairy Sci. 69:160–163.
Kirchgessner, M. and E. Grassman. 1970. Dynamics of copper absorption. In: Trace Element Metabolism in Animals (C.F. Mills, ed). Livingston, Edinburgh, pp. 227–242.
Lee, D–Y., J. Schroder and P.T. Gordon. 1988. Enhancement of copper bioavailability in the rat by phytic acid. J. Nutr. 118:712-716.
McDowell, L.R. 1992. Copper and molybdenum. In: Minerals in Animal and Human Nutrition (T.J. Cunha. ed). Academic Press, San Diego, pp. 176–204.
Miles, R.D. 1998. The influence of Eggshell 49 on shell quality of hens grouped by their shell quality. Poultry Sci. 77(Suppl. 1):43.
Powell, J.D., R. Jugdaohsingh and R.P.H. Thompson. 1999a. The regulation of mineral absorption in the gastrointestingal tract. Proc. Nutr. Soc. 58:147–153.
Powell, J.D., M.W. Whitehead, C.C. Ainley, M.D. Kendall, J.K. Nicholson and R.P.H. Thompson. 1999b. Dietary minerals in the gastrointestinal tract: hydroxypolymerisation of aluminium in regulated by luminal mucins. J. Inorg. Biochem. 75:167–180.
Rerat, A. and C. Simoes-Nunes. 1988. Amino acid absorption and production of pancreatic hormones in non-anaesthetized pigs after duodenal infusions of a milk enzymic hydrolysate or of free amino acids. Br.J. Nutr. 60:121–136.
Schultze, M.O., C.A. Elvehjem and E.B. Hart. 1934. The availability of copper in various compounds as a supplement to iron in haemoglobin formation. J. Biol. Chem. 106:735–740.
Schultze, M.O., C.A. Elvehjem and E.B. Hart. 1936. Further studies on the availability of copper from various sources as a supplement to iron in haemoglobin formation. J. Biol. Chem. 115:453-457.
Schumann, K., H.G. Classen, M. Hages, R. Prinz-Langenohl, K. Pietrzik and H.K. Biesalski. 1997. Bioavailability of oral vitamins, minerals and trace elements in perspective. Drug Res. 47:369–380.
Spain, J. 1993. Tissue integrity: a key defence against mastitis infection: the role of zinc proteinates and a theory for mode of action. In: Biotechnology in the Feed Industry (T.P. Lyons, ed). Alltech Technical Publications, Nicholasville, Kentucky, USA, pp. 53–60.
Suchy, P.E., Strakova and M. Simon. 1998. Effect of applications of various forms of zinc on gonad development in breeding cocks. Czech J. Anim. Sci. 43:343–348.
Underwood, E.J. 1997. Trace elements in human and animal nutrition. Academic Press, New York. Vallee, B.L. and K.H. Falchuk. 1993. The biological basis of zinc physiology. Physiol. Rev. 73:79-118.
Vendeland, S.C., J.T. Deagen, J.A. Butler and P.D. Whanger. 1994. Uptake of selenite, selenomethionine and selenate by brush border membrane vesicles isolated from rate small intestine. BioMetals 7:305-312.
Wapnir, R.A. 1998. Copper absorption and bioavailability. AM. J. Clin. Nutr. 67(Suppl):1054S-1060S.
Webb, K.E., J.C. Matthews and D.B. DiRienzo. 1992. Peptide absorption: A review of current concepts and future perspectives. J. Anim. Sci. 70:3248-3257.
Webb, K.E., D.B. DiRienzo and J.C. Matthews. 1993. Symposium: nitrogen metabolism and amino acid nutrition in dairy cattle. J. Dairy. Sci. 76:351-361.
Wedekind, K.J., A.E. Hortin and D.H. Baker. 1992. Methodology for assessing zinc bioavailability: efficacy estimates for zinc methionine, zinc sulphate and zinc oxide. J. Anim. Sci. 70:178-184.
Whitehead, M.W., R.P.H. Thompson and J.J. Powell. 1996. Regulation of metal absorption in the gastrointestinal tract. Gut 39:625-628.
Wolffram, S., B. Berger., B. Grenacher and E. Scharrer. 1989. Transport of selenoamino acids and their sulfur analogues across the intestinal brush border membrane of pigs. J. Nutr. 119:706-712.
Xin, Z., D.F. Waterman, R.W. Hemken and R.J. Harmon. 1991. Effects of copper status on neutrophil function, superoxide dismutase and copper distribution in steers. J.Dairy Sci. 74:3078-3082.
European Bioscience Centre, Alltech Inc., Dunboyne, Co. Meath, Ireland.






.jpg&w=3840&q=75)