Introduction
Testicular temperature of bulls must be 2 to 6° C cooler than body temperature to allow normal spermatogenesis and maturation of sperm. Exposure of bulls to increased ambient temperatures results in reduced semen quality (Casady et al., 1953; Skinner and Louw, 1966; Meyerhoeffer et al., 1985). Local heating of the scrotum of bulls for a few days causes a reduction in the number of sperm ejaculated (Austin et al., 1961; Gerona and Sikes, 1970). Heat stress has minimal effects on testicular endocrinology in bulls (Minton et al., 1981, Barth and Bowman, 1994) although heat stressed alters steroidogenesis in boars (Wettemann and Desjardins, 1979). Increased testicular temperature, whether caused by exposure to elevated ambient temperature or fever, reduces semen quality (Waites, 1970). Breeding seasons in many locations in the U.S. occur during months when bulls are exposed to elevated ambient temperatures, and semen is collected for artificial insemination during all months of the year. Potential fertility of an individual bull is more important than of an individual cow because one bull can influence pregnancy rate of 30 or 40 cows with natural mating, or thousands of cows with artificial insemination. Exposure of bulls to optimal ambient temperatures during the two months before breeding or semen collection, and during the breeding season, without even one or two days of exposure to heat stress, is essential to maximize reproductive performance.
Temperature of the Testes
Physiological mechanisms maintain testicular temperatures 2 to 6 °C less than body temperature of bulls. The scrotal skin is thin with little hair, and much vasculature, that allows for radiation and heat loss. Scrotal sweat glands also result in evaporative cooling. The tunica dartos muscle layer of the scrotum relaxes during heat stress to increase surface area. The cremaster muscle regulates the position of the testis with respect to the abdominal wall and relaxes during exposure of bulls to elevated ambient temperature. The spermatic vasculature and counter-current heat exchange is essential for reducing temperature of arterial blood from the body by transferring heat to cooler venous blood returning from the testis. These mechanisms function well to maintain cool productive testes until an animal receives an excessive heat load from either the environment or by internal heat production associated with fever.
Whole Body Exposure to Elevated Ambient Temperature and Semen Quality
A comprehensive study by Meyerhoeffer et al. (1985) evaluated the effects of whole body heat on reproductive function of sixteen yearling Angus bulls. Bulls were individually stalled in two temperaturecontrolled chambers and adjusted to 23° C for 8 wk. Then heat-stressed bulls were exposed to 35 ± 1° C for 8 h and 31 ± 1 °C for 16 h during each 24-h period for 8 wk. Control bulls were exposed to 23 ± 1 C for 8 wk. Bulls were maintained at 23 ± 1 °C after treatment for the remainder of the trial. Both groups of bulls gained about 0.6 kg/d.
All bulls were trained so that semen could be collected with an artificial vagina. Treatments were initiated after bulls were exposed to 23 °C for 8 wk and semen had been collected for 3 wk. Semen was collected twice weekly during the 8 wk of treatment and for 8 wk after treatment.
Respiratory rates were greater (P < 0.001) in bulls exposed to elevated ambient temperatures compared with control bulls (54 vs 30 breaths/min, respectively) and returned to pretreatment values within 24 h after return to thermoneutral temperatures. The effect of heat stress on respiratory rates is similar in boars (Wettemann et al., 1976). Rectal temperature was also greater in heat-stressed compared with control bulls. Bulls exposed to elevated ambient temperatures consumed 35% more water than control bulls. The increases in rectal temperature, respiratory rate, and water intake indicate that the bulls were heat stressed.
Heat stress decreased (P < 0.05) the volume of semen produced in these Angus bulls during the first 6 wk of treatment. However semen volume was not influenced by heat stress in dairy bulls (Johnson and Branton, 1953) or boars (Wettemann et al., 1976). Concentrations of testosterone in plasma of these bulls was not influenced by exposure to heat stress (Minton et al., 1981).
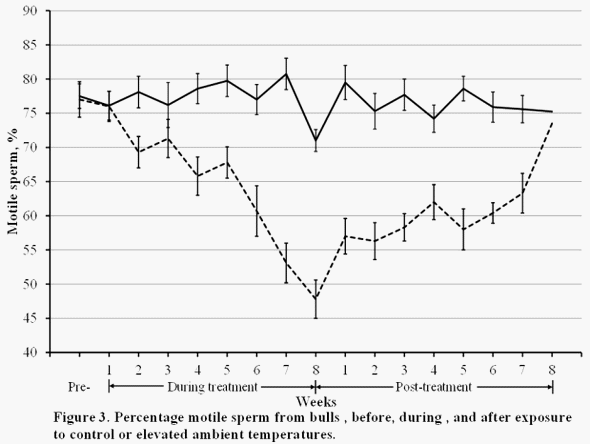
Sperm motility is a good estimate of potential fertility of bulls. The percentage of motile sperm was decreased by 2 wk after the initiation of heat stress, and after 8 wk of heat stress only 48 ± 1 % of sperm from stressed bulls were motile compared with 76 ± 2 % of sperm from control bulls (P < 0.01; Figure 1). It takes about 7 to 10 d for sperm to be transported through the epididymis of bulls, thus the lag in the time until sperm motility is decreased due to heat stress indicates that epididymidal sperm may not be affected or it takes several weeks for the change to take place in the epididymis. Several weeks were necessary for sperm motility to increase after cessation of heat stress. Sperm motility did not return to pretreatment values until 8 wk after the end of heat stress. This 8 wk lag from the end of heat stress until the return to normal motility has also been observed for Bos indicus and Bos Taurus bulls (Skinner and Louw, 1966). Total sperm output was not consistently influenced by heat stress, however stressed bulls produced more abnormal sperm (P < 0.01) and more sperm with aged acrosomes (P < 0.01). Fertility of sperm is reduced when acrosomal function is decreased (Brick et. al., 2010).
These results indicate that if bulls cannot increase the rate of heat loss from the body when they are exposed to elevated ambient temperatures, semen quality and potential fertility will be reduced. Most important in cattle breeding programs, is that 8 wk are necessary for resumption of normal semen production after bulls are exposed to elevated ambient temperatures that cause heat stress.
Acute Exposure to Elevated Ambient Temperature and Semen Quality
Although spermatogenesis is a long term process that requires about 60 d in bulls, short term heat stress is adequate to alter the process. Exposure of bulls to 40°C and 35-45% relative humidity for 12 h, 24 h, or 6 d resulted in decreased percentages of motile and live sperm and increased percentages of primary and secondary abnormalities (Skinner and Louw, 1966). Semen quality was reduced within one week after heat stress, continued to decrease for 4 wk, and returned to pretreatment values by 8 weeks after cessation of thermal stress. Total sperm output was not influenced by the treatments. When the bulls were exposed to 40°C, spermatids and spermatozoa in the final stages of development were influenced the greatest.
Insulation of the scrotum of bulls to decrease heat loss and increase testicular temperature also influences semen quality (Barth and Bowman, 1994). When the scrotum of bulls was insulated for 4 d, scrotal surface temperature was increased 1 to 1.5 °C. This resulted in decreased normal and live sperm, and increases in many types of sperm defects. The percentage normal sperm was decreased by 7 d after initiation of treatment, was minimal by 3 wk, and returned to the pretreatment percentage by 6 week after initiation of treatment. Rate of sperm passage in the epididymis was not altered by heating the testes.
Bulls can have short term increases in body temperature caused by exposure to elevated ambient temperature for several days without shade, transportation during hot weather, or infection. Short term exposure to elevated ambient temperatures must by monitored when evaluating factors that may influence bull fertility.
Management to Reduce
Heat Stress Providing shade for bulls during exposure to elevate ambient temperatures will reduce black globe temperature and reduce exposure to radiant energy. The efficiency of shades to provide relief from heat stress in the summer was evaluated in Oklahoma (Coleman et al., 1984). Bulls were exposed to ambient temperatures greater than 38 C for several weeks. There was a tendency for motility and the percentage of live sperm cells to be greater, and the percentage of abnormal sperm to be reduced when bulls had access to shade. Thus access to shade has the potential to improve fertility of bulls during the summer.
When Does Ambient Temperature Become Heat Stress?
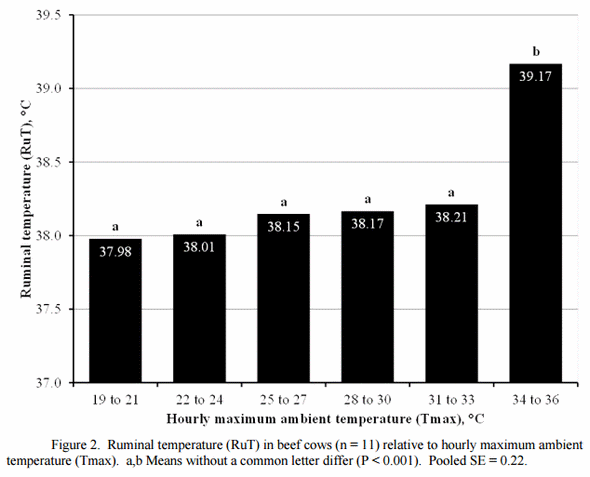
Cattle can remove excessive heat from their bodies by sweating and panting until the mechanisms become inadequate to transfer heat to the environment. We evaluated the effect of ambient temperature on ruminal temperature of mature cows in Oklahoma in early summer (Boehmer, 2011). Cows were maintained in a drylot with ad libitum hay, water and shade. Cows maintained normal ruminal temperature (RuT) when ambient temperature was less than 32°C (90°F). However when ambient temperature was ≥ 32°C, RuT increased (Figure 2). Ruminal temperature was increased 1°C greater than normal when ambient temperature was increased to 35°C. Similarily, rectal temperature was increase when ambient temperature was ≥ 31°C in cattle (Casady et al., 1956; Pratt and Wettemann, 1986).
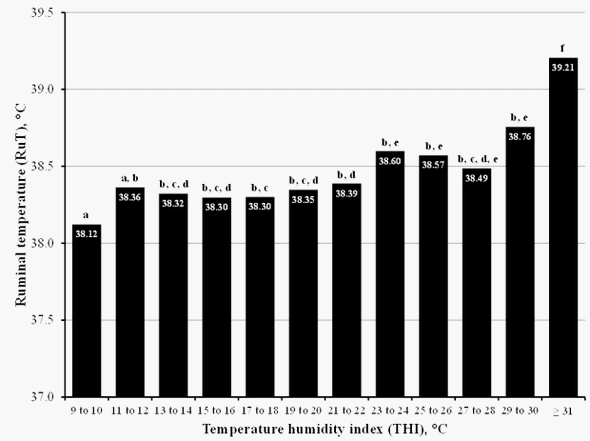
Heat loss from cattle depends on evaporative cooling from the skin associated with sweating or from the respiratory passage. In either case, the relative humidity influences the rate of evaporative cooling. The effect of elevated ambient temperature on heat loss from cattle near the Gulf Coast would be very different compared with west Texas because of differences in relative humidity. The temperature humidity index (THI = (0.8 x temp) +(% rel humid/100) x ( temp –14.4) +46.6) adjusts the effect of temperature on cattle for the relative humidity (Thom, 1959). Cows maintained normal ruminal temperature (RuT) when THI ambient temperature was less than 31°C (90°F). However, when THI ≥ 31°C RuT increased (Figure 3). Ruminal temperature was increased 1.5°C greater than normal when THI was increased from 31 to 34°C. Both THI and ambient temperature were correlated (r = 0.95) with RuT in the cows. This information with mature cows indicates that exposure of bulls to greater than 30°C has the potential to reduce semen quality.
Although it is not well documented in cattle, male swine may be more susceptible to exposure to elevated ambient temperatures than females. Exposed of gilts to heat stress during early gestation (d 0 to 16) or late gestation (d 102 to 110) reduced reproductive performance. However when gilts were exposed to elevated ambient temperatures during mid-gestation (d 53 to 61) reproductive performance was not altered (Omtvedt et al., 1971). Spermatogenesis is a continuous process and boars are constantly sensitive to exposure to elevated ambient temperature, and may be more sensitive to lesser increases in ambient temperature than gilts (Wettemann et al., 1976). Because bulls are always sensitive to exposure to elevated ambient temperature, semen quality may be reduced by exposure to minimal increases in ambient temperature. One bull influences pregnancy rate associated with numerous matings. Thus maintaining bulls in a cool environment should be a high priority to achieve good pregnancy rates.
References
Austin, J.W., E.W. Hupp, and R.L. Murphree. 1961. Effect of scrotal insulation on semen of Hereford bulls. J. Anim. Sci. 20:307.
Barth, A.D. and P.A. Bowman. 1994. The sequential appearance of sperm abnormalities after scrotal insulation of dexamethasone treatment of bulls. Can. Vet. J. 34:93-102.
Boehmer, B.H., T.A. Pye, and R.P. Wettemann. 2011. Effect of ambient temperature on ruminal temperature in beef cows. J. Anim. Sci. (E-Suppl. 2)89:141.
Birck A., P. Christensen, R. Labouriau, J. Pedersen, and S. Borchersen. 2010. In vitro induction of the acrosome reaction in bull sperm and the relationship to field fertility using low-dose inseminations. Theriogenoloty 73:1180-1191.
Casady, R.B., R.M. Meyers, and J.E. Legates. 1953. The effect of exposure to high ambient temperature on spermatogenesis in the dairy bull. J. Dairy Sci. 36:14.
Casady, R.B., J.E. Legates, and R.M. Myers. 1956. Correlations between ambient temperatures varying from 60°-95° and certain physiological responses in yung dairy bulls. J. Anim. Sci. 15:141-152.
Coleman, S.W., D.C. Meyerheoffer, and F.P. Horn. 1984. Semen characteristics and behavior of grazing bulls as influenced by shade. J. Range Management 37:243-247.
Gerona, G. R. and J.D. Sikes. 1970. Effects of elevated scrotum temperature on spermatogenesis and semen characteristics. J. Dairy Sci. 53:659.
Johnson, J.E. and C. Branton. 1953. Effects of seasonal climatic changes on certain physiological reactions, semen production and fertility of dairy bulls. J. Dairy Sci. 36:934-942.
Meyerhoeffer, D.C., R.P. Wettemann, S.W. Coleman and M.E. Wells. 1985. Reproductive criteria of beef bulls during and after exposure to increased ambient temperature. J. Anim. Sci. 60:352-357.
Minton, J.E., R.P. Wettemann, D.C. Meyerhoeffer, R.L. Hintz and E.J. Turman. 1981. Serum luteinizing hormone and testosterone in bulls during exposure to elevated ambient temperature. J. Anim. Sci. 53:15511558.
Omtvedt, I.T., R.E. Nelson, R.L. Edwards, D.F. Stephens, and E.J. Turman. 1971. Influence of heat stress during early, mid and late pregnancy of gilts. J. Anim. Sci. 32:312-317.
Pratt, B.R. and R.P. Wettemann. 1986. The effect of environmental temperature on the concentrations of thyroxin and triiodothyronine after thyrotropin releasing hormone in steers. J. Anim. Sci. 62:1346-1352.
Skinner, J.D. and G.N. Louw. 1966. Heat Stress and spermatogenesis in Bos indicus and Bos Taurus cattle. J. Appl. Physiol. 21:1784-1790. 263 Thom, E. C. 1959. The discomfort index. Weatherwise 12:57–59.
Waites, G.M.H. 1970. Temperature regulation and the testis. In: Johnson A.S., W.R. Gomes, N.L. Vandemark, editors, The testis. Academic Press, New York. P. 241-279.
Wettemann, R.P., M.E. Wells, I.T. Omtvedt, C.E. Pope and E.J. Turman. 1976. Influence of elevated ambient temperature on reproductive performance of boars. J. Anim. Sci. 42:664-669.
Wettemann, R.P. and C. Desjardins. 1979. Testicular function in boars exposed to elevated ambient temperature. Biol. Reprod. 20:235-241.









