Genomic Epidemiology of Salmonella Infantis in Ecuador: From Poultry Farms to Human Infections
Author details:
Salmonella enterica is one of the most important foodborne pathogens around the world. In the last years, S. enterica serovar Infantis has become an important emerging pathogen in many countries, often as multidrug resistant clones. To understand the importance of S. enterica in the broiler industry in Ecuador, we performed a study based on phenotypic and WGS data of isolates from poultry farms, chicken carcasses and humans. We showed a high prevalence of S. enterica in poultry farms (41.4%) and chicken carcasses (55.5%), but a low prevalence (1.98%) in human samples. S. Infantis was shown to be the most prevalent serovar with a 98.2, 97.8, and 50% in farms, foods, and humans, respectively, presenting multidrug resistant patterns. All sequenced S. Infantis isolates belonged to ST32. For the first time, a pESI-related megaplasmid was identified in Ecuadorian samples. This plasmid contains genes of antimicrobial resistance, virulence factors, and environmental stress tolerance. Genomic analysis showed a low divergence of S. Infantis strains in the three analyzed components. The results from this study provide important information about genetic elements that may help understand the molecular epidemiology of S. Infantis in Ecuador.
Keywords: Salmonella Infantis, ST32, broiler, WGS, Ecuador, megaplasmid, multidrug resistance (MDR)
INTRODUCTION
MATERIALS AND METHODS
Study Design and Sampling
Isolation and Identification of Salmonella enterica
Antimicrobial Susceptibility Testing
Detection of Extended Spectrum Beta-Lactamase (ESBL) Genes
Whole Genome Sequencing
Serotype Identification
MLST Analysis, Antimicrobial Resistance Genes, and Plasmid Detection
Megaplasmid Analysis
Core Genome and Metadata Analysis
RESULTS
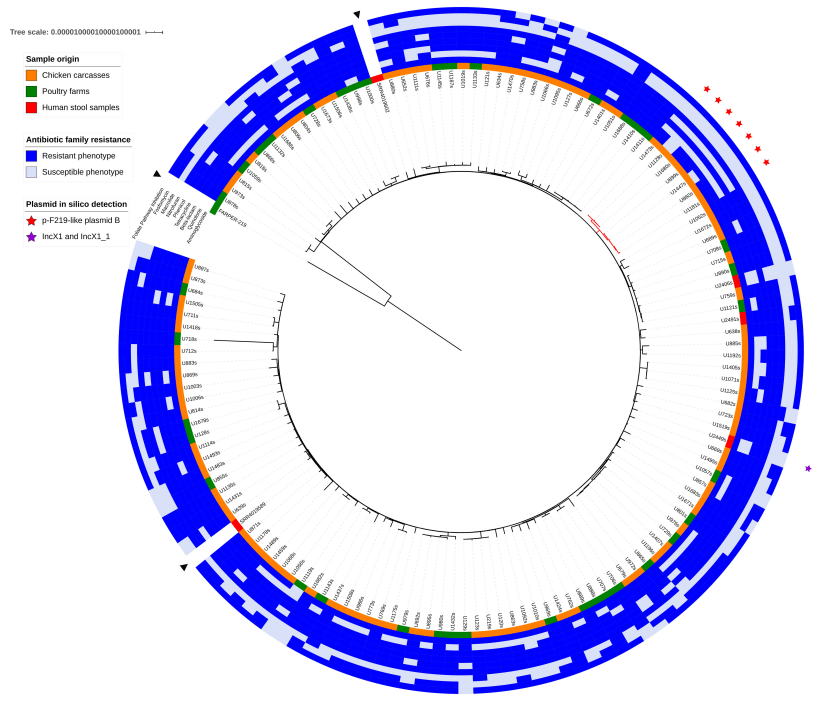
Salmonella Prevalence and Serotype Identification
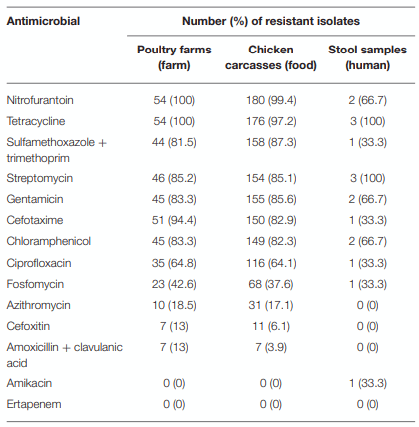
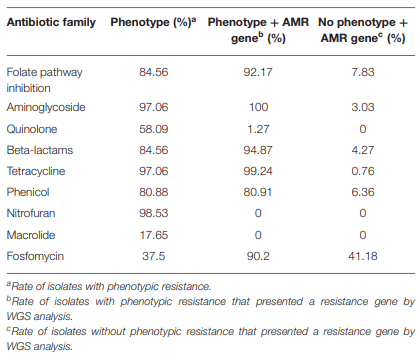
Antimicrobial Resistance
![TABLE 2 | Antimicrobial resistance patterns of S. Enteritidis, S. Typhimurium, and monophasic S. Typhimurium 4,[5],12:i:-.](/_next/image/?url=https%3A%2F%2Fimages.engormix.com%2FE_articles%2F53674_257.gif&w=1080&q=75)
Genomic Analysis
DISCUSSION
DATA AVAILABILITY STATEMENT
ETHICS STATEMENT
AUTHOR CONTRIBUTIONS
FUNDING
ACKNOWLEDGMENTS
SUPPLEMENTARY MATERIAL
1. WHO. WHO Estimates of the Global Burden of Foodborne Diseases. Foodborne Disease Burden Epidemiolgy Reference Group 2007–2015. Geneva: World Heal Organization (2015). p. 1–15.
2. Antunes P, Mourão J, Campos J, Peixe L. Salmonellosis: the role of poultry meat. Clin Microbiol Infect. (2016) 22:110–21. doi: 10.1016/j.cmi.2015.12.004
3. Koutsoumanis K, Allende A, Alvarez-Ordóñez A, Bolton D, Bover-Cid S, Chemaly M, et al. Salmonella control in poultry flocks and its public health impact. EFSA J. (2019) 17:e05596. doi: 10.2903/j.efsa.2019.5596
4. CONAVE. Estadísticas Del Sector Avícola. (2020) Available online at: https:// www.conave.org/informacion-sector-avicola-publico/ (accessed February 21, 2020).
5. Donado-Godoy P, Byrne BA, León M, Castellanos R, Vanegas C, Coral A, et al. Prevalence, resistance patterns, and risk factors for antimicrobial resistance in bacteria from retail chicken meat in Colombia. J Food Prot. (2015) 78:751–9. doi: 10.4315/0362-028X.JFP-14-349
6. Valderrama W, Pastor J, Mantilla Salazar J, Ortiz M. Estudio de prevalencia de serotipos de salmonella en granjas avícolas tecnificadas en el perú. Lima. (2014).
7. Vinueza-Burgos C, Baquero M, Medina J, De Zutter L. Occurrence, genotypes and antimicrobial susceptibility of salmonella collected from the broiler production chain within an integrated poultry company. Int J Food Microbiol. (2019) 299:1–7. doi: 10.1016/j.ijfoodmicro.2019.03.014
8. Vinueza-Burgos C, Cevallos M, Ron-Garrido L, Bertrand S, De Zutter L. Prevalence and diversity of salmonella serotypes in ecuadorian broilers at slaughter age. PLoS ONE. (2016) 11:e0159567. doi: 10.1371/journal.pone.0159567
9. Voss-Rech D, Vaz CSL, Alves L, Coldebella A, Leao JA, Rodrigues DP, et al. A temporal study of salmonella enterica serotypes from broiler farms in Brazil. Poult Sci. (2015) 94:433–41. doi: 10.3382/ps/peu081
10. Brown AC, Chen JC, Watkins LKF, Campbell D, Folster JP, Tate H, et al. CTX-M-65 extended-spectrum β-lactamase–producing salmonella enterica serotype infantis, united states1. Emerg Infect Dis. (2018) 24:2284–91. doi: 10.3201/eid2412.180500
11. Cartelle Gestal M, Zurita J, Paz y Mino A, Ortega-Paredes D, Alcocer I. Characterization of a small outbreak of salmonella enterica serovar infantis that harbour CTX-M-65 in ecuador. Brazilian J Infect Dis. (2016) 20:406–7. doi: 10.1016/j.bjid.2016.03.007
12. Tate H, Folster JP, Hsu C-H, Chen J, Hoffmann M, Li C, et al. Comparative analysis of extended-spectrum-β-lactamase CTX-M-65-producing salmonella enterica serovar infantis isolates from humans, food animals, and retail chickens in the united states. Antimicrob Agents Chemother. (2017) 61:1–11. doi: 10.1128/AAC.00488-17
13. McDermott PF, Zhao S, Tate H. Antimicrobial resistance in nontyphoidal salmonella. Microbiol Spectr. (2018) 6:780–90. doi: 10.1128/9781555819804.ch12
14. Ibarra JA, Steele-Mortimer O. Salmonella - the ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cell Microbiol. (2009) 11:1579–86. doi: 10.1111/j.1462-5822.2009.01368.x
15. WHO. Integrated Surveillance of Antimicrobial Resistance in Foodborne Bacteria. (2017). Available online at: http://apps.who.int/iris/bitstream/10665/ 91778/1/9789241506311_eng.pdf (accessed July 25, 2020).
16. Akiba M, Kusumoto M, Iwata T. Rapid identification of salmonella enterica serovars, typhimurium, choleraesuis, infantis, hadar, enteritidis, dublin and gallinarum, by multiplex PCR. J Microbiol Methods. (2011) 85:9–15. doi: 10.1016/j.mimet.2011.02.002
17. CLSI (Clinical Laboratory Standards Institute). Performance standards for antimicrobial susceptibility testing; twenty-nine informational supplement. In: Weinstein MP, editor. CLSI Document M100-S29. 29th ed. Wayne: Clinical and Laboratory Standards Institute. (2019). p. 32–41.
18. Hasman H, Mevius D, Veldman K, Olesen I, Aarestrup FM. β-Lactamases among extended-spectrum beta-lactamase (ESBL)-resistant Salmonella from poultry, poultry products and human patients in The Netherlands. J Antimicrob Chemother. (2005) 56:115–21. doi: 10.1093/jac/dki190
19. Olesen I, Hasman H, Aarestrup FM. Prevalence of beta-lactamases among ampicillin-resistant Escherichia coli and salmonella isolated from food animals in Denmark. Microb Drug Resist. (2004) 10:334–40. doi: 10.1089/mdr.2004.10.334
20. Kruger T, Szabo D, Keddy KH, Deeley K, Marsh JW, Hujer AM, et al. Infections with nontyphoidal salmonella species producing TEM63 or a novel TEM enzyme, TEM-131, in South Africa. Antimicrob Agents Chemother. (2004) 48:4263–70. doi: 10.1128/AAC.48.11.4263-427 0.2004
21. Arlet G, Rouveau M, Philippon A. Substitution of alanine for aspartate at position 179 in the SHV-6 extended-spectrum beta-lactamase. FEMS Microbiol Lett. (1997) 152:163–7. doi: 10.1016/S0378-1097(97)00196-1
22. Carattoli A, García-Fernández A, Varesi P, Fortini D, Gerardi S, Penni A, et al. Molecular epidemiology of Escherichia coli producing extended-spectrum beta-lactamases isolated in Rome, Italy. J Clin Microbiol. (2008) 46:103–8. doi: 10.1128/JCM.01542-07
23. Jiang X, Zhang Z, Li M, Zhou D, Ruan F, Lu Y. Detection of extendedspectrum beta-lactamases in clinical isolates of pseudomonas aeruginosa. Antimicrob Agents Chemother. (2006) 50:2990–5. doi: 10.1128/AAC.01511-05
24. Hopkins KL, Batchelor MJ, Liebana E, Deheer-Graham AP, Threlfall EJ. Characterisation of CTX-M and AmpC genes in human isolates of Escherichia coli identified between 1995 and 2003 in England and Wales. Int J Antimicrob Agents. (2006) 28:180–92. doi: 10.1016/j.ijantimicag.2006.03.027
25. Paauw A, Fluit AC, Verhoef J, Leverstein-van Hall MA. Enterobacter cloacae outbreak and emergence of quinolone resistance gene in Dutch hospital. Emerg Infect Dis. (2006) 12:807–12. doi: 10.3201/eid1205.050910
26. Dierikx CM, van Duijkeren E, Schoormans AHW, van Essen-Zandbergen A, Veldman K, Kant A, et al. Occurrence and characteristics of extendedspectrum-β-lactamase- and AmpC-producing clinical isolates derived from companion animals and horses. J Antimicrob Chemother. (2012) 67:1368–74. doi: 10.1093/jac/dks049
27. Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. (2012) 67:2640–4. doi: 10.1093/jac/dks261
28. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. (2014) 30:2114–20. doi: 10.1093/bioinformatics/btu170
29. Wingett SW, Andrews S. FastQ screen: a tool for multi-genome mapping and quality control. F1000Research. (2018) 7:1338. doi: 10.12688/f1000research.15931.2
30. Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. (2016) 32:3047–8. doi: 10.1093/bioinformatics/btw354
31. Zhang S, Yin Y, Jones MB, Zhang Z, Deatherage Kaiser BL, Dinsmore BA, et al. Salmonella serotype determination utilizing high-throughput genome sequencing data. J Clin Microbiol. (2015) 53:1685–92. doi: 10.1128/JCM.00323-15
32. Hunt M, Mather AE, Sánchez-Busó L, Page AJ, Parkhill J, Keane JA, et al. ARIBA: rapid antimicrobial resistance genotyping directly from sequencing reads. Microb Genomics. (2017) 3:e000131. doi: 10.1099/mgen.0.000131
33. Jolley KA, Bray JE, Maiden MCJ. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. (2018) 3:124. doi: 10.12688/wellcomeopenres.14826.1
34. Carattoli A, Zankari E, Garciá-Fernández A, Larsen MV, Lund O, Villa L, et al. In silico detection and typing of plasmids using plasmidfinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. (2014) 58:3895–903. doi: 10.1128/AAC.02 412-14
35. Vallejos-Sánchez K, Tataje-Lavanda L, Villanueva-Pérez D, Bendezú J, Montalván Á, Zimic-Peralta M, et al. Whole-genome sequencing of a salmonella enterica subsp. Enterica serovar infantis strain isolated from broiler chicken in Peru. Microbiol Resour Announc. (2019) 8:e00826–19. doi: 10.1128/MRA.00826-19
36. Li H, Durbin R. Fast and accurate long-read alignment with burrows-wheeler transform. Bioinformatics. (2010) 26:589–95. doi: 10.1093/bioinformatics/btp698
37. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. 1000 genome project data processing subgroup. The sequence alignment/map format and SAMtools. Bioinformatics. (2009) 25:2078–9. doi: 10.1093/bioinformatics/btp352
38. Li H. Seqtk: Toolkit for Processing Sequences in FASTA/Q Formats Hinxton. (2012).
39. Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. (2020) 37:1530–34. doi: 10.1101/849372
40. Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. (2014) 30:2068–9. doi: 10.1093/bioinformatics/btu153
41. Lechner M, Findeiss S, Steiner L, Marz M, Stadler PF, Prohaska SJ. Proteinortho: detection of (co-)orthologs in large-scale analysis. BMC Bioinformatics. (2011) 12:124. doi: 10.1186/1471-2105-12-124
42. Cabanettes F, Klopp C. D-GENIES: dot plot large genomes in an interactive, efficient and simple way. PeerJ. (2018) 6:e4958. doi: 10.7717/peerj.4958
43. Darling AE, Mau B, Perna NT. Progressivemauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE. (2010) 5:e11147. doi: 10.1371/journal.pone.0011147
44. Yoon S-H, Ha S-M, Lim J, Kwon S, Chun J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek. (2017) 110:1281–6. doi: 10.1007/s10482-017-0844-4
45. Nurk S, Bankevich A, Antipov D, Gurevich A, Korobeynikov A, Lapidus A, et al. Assembling genomes mini-metagenomes from highly chimeric reads. In: M Deng, R Jiang, F Sun, X Zhang, editors. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence Lecture Notes in Bioinformatics). Berlin: Springer (2011). p. 158–170. doi: 10.1007/978-3-642-37195-0_13
46. Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast fourier transform. Nucleic Acids Res. (2002) 30:3059–66. doi: 10.1093/nar/ gkf436
47. Letunic I, Bork P. Interactive tree of life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. (2019) 47:W256–9. doi: 10.1093/nar/gkz239
48. Hendriksen RS, Vieira AR, Karlsmose S, Lo Fo Wong DMA, Jensen AB, Wegener HC, et al. Global monitoring of salmonella serovar distribution from the world health organization global foodborne infections network country data bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog Dis. (2011) 8:887–900. doi: 10.1089/fpd.201 0.0787
49. EFSA (European Food Safety Authority), ECDC (European Centre for Disease Prevention and Control). The European union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2012. EFSA J. (2014) 12:e5077. doi: 10.2903/j.efsa.2014. 3547
50. Gymoese P, Kiil K, Torpdahl M, Østerlund MT, Sørensen G, Olsen JE, Nielsen EM, et al. WGS based study of the population structure of salmonella enterica serovar infantis. BMC Genomics. (2019) 20:870. doi: 10.1186/s12864-019-6260-6
51. Donado-Godoy P, Gardner I, Byrne BA, Leon M, Perez-Gutierrez E, Ovalle MV, et al. Prevalence, risk factors, and antimicrobial resistance profiles of salmonella from commercial broiler farms in two important poultry-producing regions of Colombia. J Food Prot. (2012) 75:874–83. doi: 10.4315/0362-028X.JFP-11-458
52. Villagomez S. Aislamiento y serotipificación de salmonella enteritidis, typhimurium e infantis en carcasas de pollo destinadas para consumo humano en un camal industrializado de la provincia de pichincha Quito: Universidad Central del Ecuador. (2015)
53. Vasco G, Trueba G, Atherton R, Calvopiña M, Cevallos W, Andrade T, et al. Identifying etiological agents causing diarrhea in low income ecuadorian communities. Am J Trop Med Hyg. (2014) 91:563–9. doi: 10.4269/ajtmh.13-0744
54. Naranjo A, Cedeño C, Teran E, Castello A, CASERO Research Team. Prevalence of VP4 and VP7 genotypes of human rotavirus in ecuadorian children with acute diarrhea. J Med Virol. (2008) 80:1106–11. doi: 10.1002/jmv.21181
55. Guderian RH, Ordóñez G, Bossano R. Acute diarrhea associated with a campylobacter and other pathogenic agents in Quito, Ecuador. Boletín Of Sanit Panam. (1987) 102:333–9.
56. Bhavnani D, Goldstick JE, Cevallos W, Trueba G, Eisenberg JNS. Synergistic effects between rotavirus and coinfecting pathogens on diarrheal disease: evidence from a community-based study in northwestern ecuador. Am J Epidemiol. (2012) 176:387–95. doi: 10.1093/aje/kws220
57. Gunn JS, Marshall JM, Baker S, Dongol S, Charles RC, Ryan ET. Salmonella chronic carriage: epidemiology, diagnosis, and gallbladder persistence. Trends Microbiol. (2014) 22:648–55. doi: 10.1016/j.tim.2014.06.007
58. Food E, Authority S. The European union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. (2018) 16:e05500. doi: 10.2903/j.efsa.2018.5500
59. Boscán-Duque L, Arzálluz-Fisher A, Ugarte C, Sánchez D, Wittum T, Hoet A. Reduced susceptibility to quinolones among salmonella serotypes isolated from poultry at slaughter in venezuela. J Food Prot. (2007) 70:2030–35. doi: 10.4315/0362-028X-70.9.2030
60. Medeiros MAN, de Oliveira DCN, Rodrigues DDP, de Freitas DRC. Prevalence and antimicrobial resistance of salmonella in chicken carcasses at retail in 15 Brazilian cities. Rev Panam Salud Publica. (2011) 30:555–60. doi: 10.1590/S1020-49892011001200010
61. Lapierre L, Cornejo J, Zavala S, Galarce N, Sánchez F, Benavides MB, et al. Phenotypic and genotypic characterization of virulence factors and susceptibility to antibiotics in salmonella infantis strains isolated from chicken meat: first findings in chile. Animals. (2020) 10:1049. doi: 10.3390/ani10061049
62. Foley SL, Nayak R, Hanning IB, Johnson TJ, Han J, Ricke SC. Population dynamics of salmonella enterica serotypes in commercial egg and poultry production. Appl Environ Microbiol. (2011) 77:4273–9. doi: 10.1128/AEM.00598-11
63. Da Cunha-Neto A, Carvalho LA, Carvalho RCT, Dos Prazeres Rodrigues D, Mano SB, De Souza Figueiredo EE, et al. Salmonella isolated from chicken carcasses from a slaughterhouse in the state of mato grosso, Brazil: antibiotic resistance profile, serotyping, and characterization by repetitive sequencebased PCR system. Poult Sci. (2018) 97:1373–81. doi: 10.3382/ps/pex406
64. Quesada A, Reginatto GA, Español AR, Colantonio LD, Burrone MS. Resistencia antimicrobiana de salmonella spp aislada de alimentos de origen animal para consumo humano. Rev Peru Med Exp Salud Publica. (2016) 33:32–44. doi: 10.17843/rpmesp.2016.331.1899
65. Rodriguez R, Fandiño C, Donado P, Guzmán L, Verjan N. Characterization of salmonella from commercial egg-laying hen farms in a central region of Colombia. Avian Dis. (2015) 59:57–63. doi: 10.1637/10873-052714-Reg
66. EFSA. The European union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA J. (2020) 18:e06007. doi: 10.2903/j.efsa.2020.6007
67. Vinueza C. Salmonella and Campylobacter in Broilers at Slaughter Age: A Possible Source for Carcasses Contamination in Ecuador. Merelbeke: Faculty of Veterinary Medicine, Ghent University. (2017).
68. Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin S a, Robinson TP, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA. (2015) 112:5649–54. doi: 10.1073/pnas.1503141112
69. Hindermann D, Gopinath G, Chase H, Negrete F, Althaus D, Zurfluh K, et al. Salmonella enterica serovar infantis from food and human infections, Switzerland, 2010-2015: poultry-related multidrug resistant clones and an emerging ESBL producing clonal lineage. Front Microbiol. (2017) 8:1322. doi: 10.3389/fmicb.2017.01322
70. Bogomazova AN, Gordeeva VD, Krylova EV, Soltynskaya IV, Davydova EE, Ivanova OE, et al. Mega-plasmid found worldwide confers multiple antimicrobial resistance in salmonella infantis of broiler origin in Russia. Int J Food Microbiol. (2020) 319:108497. doi: 10.1016/j.ijfoodmicro.2019.108497
71. Wajid M, Saleemi MK, Sarwar Y, Ali A. Detection and characterization of multidrug-resistant salmonella enterica serovar infantis as an emerging threat in poultry farms of faisalabad, Pakistan. J Appl Microbiol. (2019) 127:248–61. doi: 10.1111/jam.14282
72. Vuthy Y, Lay KS, Seiha H, Kerleguer A, Aidara-Kane A. Antibiotic susceptibility and molecular characterization of resistance genes among Escherichia coli and among salmonella subsp. in chicken food chains. Asian Pac J Trop Biomed. (2017) 7:670–4. doi: 10.1016/j.apjtb.2017.07.002
73. Aviv G, Tsyba K, Steck N, Salmon-Divon M, Cornelius A, Rahav G, et al. A unique megaplasmid contributes to stress tolerance and pathogenicity of an emergent salmonella enterica serovar infantis strain. Environ Microbiol. (2014) 16:977–94. doi: 10.1111/1462-2920.12351
74. Franco A, Leekitcharoenphon P, Feltrin F, Alba P, Cordaro G, Iurescia M, et al. Emergence of a clonal lineage of multidrug-resistant ESBLproducing salmonella infantis transmitted from broilers and broiler meat to humans in Italy between 2011 and 2014. PLoS ONE. (2015) 10:e0144802. doi: 10.1371/journal.pone.0144802
75. Cohen E, Rahav G, Gal-Mor O. Genome sequence of an emerging salmonella enterica serovar infantis and genomic comparison with other S. infantis strains. Genome Biol Evol. (2020)12:223–8. doi: 10.1093/gbe/evaa048
76. Ledeboer NA, Frye JG, McClelland M, Jones BD. Salmonella enterica serovar typhimurium requires the Lpf, Pef, and Tafi fimbriae for biofilm formation on HEp-2 tissue culture cells and chicken intestinal epithelium. Infect Immun. (2006) 74:3156–69. doi: 10.1128/IAI.01428-05
77. Kingsley RA, Weening EH, Keestra AM, Bäumler AJ. Population heterogeneity of salmonella enterica serotype typhimurium resulting from phase variation of the lpf operon in vitro and in vivo. J Bacteriol. (2002) 184:2352–9. doi: 10.1128/JB.184.9.2352-235 9.2002
78. Gupta SK, Sharma P, McMillan EA, Jackson CR, Hiott LM, Woodley T, et al. Genomic comparison of diverse salmonella serovars isolated from swine. PLoS ONE. (2019) 14:e0224518. doi: 10.1371/journal.pone.02 24518
79. Boddicker JD, Ledeboer NA, Jagnow J, Jones BD, Clegg S. Differential binding to and biofilm formation on, HEp-2 cells by salmonella enterica serovar typhimurium is dependent upon allelic variation in the fimH gene of the fim gene cluster. Mol Microbiol. (2002) 45:1255–65. doi: 10.1046/j.1365-2958.2002. 03121.x