I. DEVELOPMENT OF A BALANCED GUT MICROBIOTA ECOSYSTEM AND THE COMPLEX HOST –MICROBIOTA INTERACTION.
In the past, investigation of the intestinal bacterial population has been done with in vitro culture techniques. These techniques are able to assess only those bacterial species that can be grown in different media in laboratory conditions. Modern approaches using molecular techniques were able to show that a significant part of intestinal microbiota was not properly assessed. Any conclusions regarding the composition of intestinal microbiota and its functions must be drawn very carefully. Use of modern molecular techniques has led to a better understanding of the role of microbiota in oral tolerance and physiological functions of a healthy gut and has helped a lot to disqualify simplistic views such as the existing of ‘good’ Lactobacillus spp. and ‘bad’ Clostridium spp. bacteria.
Nowadays, microbiota is considered as a gene toolbox that is complementing the gene pool of the host. The research is focusing on unravelling the complex interactions of what kind of gene pool is linked with good gut health and understanding how genes, both from the gut and its microbiota, can be switched on and off with different diet types, in order to reach the best performance: lowest level of inflammation, best digestive and absorptive properties, What we know, so far, is that a composition of intestinal microbiota is changing throughout the life cycle of an individual, becoming quantitatively and qualitatively more complex with age. Also, environmental factors such as stocking density, diet composition and feeding practice, management, housing conditions, pathogen load in the environment, use of antibiotics can modify intestinal microbiota. Feed withdrawal, especially over a longer time, causes reduction in the number of detected bacterial species. Also, from one segment of the gastro-intestinal tract to another, bacterial populations of the gut vary significantly. In the small intestine of a healthy bird, Lactobacillaceae spp. are dominant, whereas in caeca Clostrideaceae spp. are prevailing, which is connected with different pH and physiologic functions of these intestinal segments.
There are some members of the mucosa-associated microbial community that are considered to be especially crucial for a healthy status of the gut such as Ruminococcaceae and Lachnospiraceae. Bacteria producing short chain fatty acids, like acetic, propionic and butyric acid, during the fermentation process of dietary carbohydrates are considered supportive for a good gut health. Production of butyrate near the epithelial cells and in close association with potentially invading and histotoxic pathogens promotes development and recovery of the villi, stimulates the expression of the tight junction proteins, limits invasion of potential pathogens such as E. coli and Salmonella spp. and further promotes a beneficial microbial ecosystem, which leads to an overall increased tissue health. On the contrary, mucin-desulphating bacteria and sulphate reducers create hydrogen sulphide, which enhances some pathogens and causes tissue damage. Lipopolysaccharide (LPS) containing Enterobacteriaceae are generally considered as linked with negative gut health status.
When judging feed composition, management actions, additives, anticoccidial, antiviral or other strategies that aim to reduce (the impact of) BE, assessing intestinal microbiome profiling can now be performed more easily for instance by next-generation sequencing of 16S ribosomal DNA. As important, also the host reaction to microbiota changes can now be more easily investigated for instance by evaluating mRNA expression of expression of genes involved in the mitogen-activated protein kinase pathway.
II. VICIOUS CIRCLE OF BACTERIAL ENTERITIS
Since the ban of antimicrobial growth promoters in Europe in 2006, broiler bacterial enteritis (BE) issues have increased in many production units. The aetiology of BE is multifactorial. In modern broiler breeds, selected for maximal growth rate and high feed intake, abundance of non-absorbed nutrients in the gut lumen, in absence of growth promoters with antibacterial properties, causes a chain of events that exacerbates the proliferation of some clusters of bacteria that leads to an inflammatory reaction of the gut wall. This reaction of the gut wall in turn instigates microscopic and macroscopic changes that, as in a vicious circle, will lead to poorer physiologic status of the intestine and to poor digestive and absorptive functions, resulting in even more nutrients in the intestinal lumen, and more substrate for bacterial growth. These macroscopic signs have led to a scoring system that has the advantage it can be used in field conditions with immediate feedback to veterinarians in terms of treatment, versus histology that typically would require a long time for conclusions. On top of this, the macroscopic system for BE can be combined with coccidiosis lesion scoring. Still, innovation on diagnostics is expected to yield within the next couple of years of biomarker-based tests that can support and facilitate diagnosis in field conditions (De Meyer, 2019).
Figure 1 - (Maarten De Gussem, 2010). Macroscopic dysbacteriosis score system parameters. A. Overall gut ballooning; B. Content of the intestinal tract, 1. Mucoid, orange intestinal content, 2. Foamy intestinal content; C. Tonus of the intestinal tract, 1. Good tonus, 2. Lack of tonus; D. Macroscopically visible thickness of the intestinal tract, 1. Macroscopically thin intestinal tract, 2. Intestinal tract with normal thickness; E. Undigested particles in the colon (arrows); F. Inflammation of the gut, 1. Inflammation, 2. No inflammation.
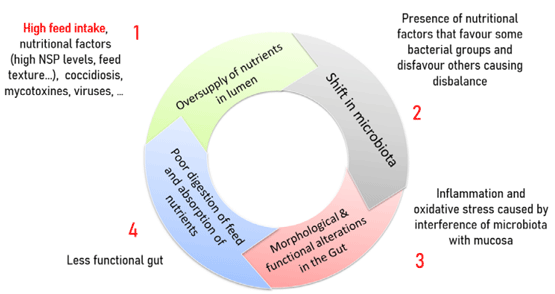
The pathogenesis of BE can be described as a vicious circle in 4 steps:
In the first step, the shift of the healthy gut towards BE starts with oversupply of nutrients in the intestinal lumen. In today’s broiler, the very high feed intake has accelerated the general feed passage rate in the intestine. So, even minor violations of the digestion and absorption will lead to an increase of the number of nutrients, especially of undigested proteins and high-energy nutrient particles in the hind gut. Among gut damaging factors (often of infectious origin), the so-called BE-instigators (BEI), coccidiosis is considered to be the most important, but also virus infections such as Reo can destroy intestinal epithelia, shorten intestinal villi and lead to poor absorption of the intestine. The stressors of non-infectious origin are dietary changes, nutritional imbalance for instance on crude protein level, soluble non-starch polysaccharides (NSP), enzymatic dysfunctions, mycotoxins and management issues.
As a consequence of the oversupply of nutrients in the intestinal lumen, a shift in proliferation of some clusters of bacteria occurs in the small intestine in step 2 of the vicious circle. The presence of excessive nutritional factors mainly favours the proliferation of Clostridium perfringens and Lactobacilli and disfavours certain Clostridiales such as Ruminococcaceae and Lachnospiraceae.
In step 3, this disruption of the delicate balance in gut microbial constellation, in an already damaged (by BEI) gut, shifts intestinal immune tolerance towards pathological inflammation reactions and oxidative stress in the gut wall, so morphological and functional alterations in the intestine occur. In case of overgrowth of Clostridium perfringens spp. producing Netβ toxin, the two first steps are very similar but these alterations result in necrotic enteritis, with a further toxin - and host related destruction of the intestinal lining.
Step 4 of the vicious circle of BE is characterised by poor digestion of feed and poor absorption of nutrients, as the damaged intestine is not able to fulfil its functions while the feed intake continues to be higher than what can be digested and absorbed due to the poor activation of MGBA negative feedback on feed intake in case of inflammation.
The above described 4 steps of the vicious circle result in a less functioning gut, which in turn leads to further oversupply of nutrients in the intestinal lumen, further reinforcing the vicious circle of BE. On a flock level, there will be flattening of the feed intake, so there will be birds that face a MGBA activation leading to lower feed intake, but that comes usually too late as these birds have already tumbled into the vicious circle. Mostly these flocks will have a further increase of water intake, so this typically leads to increased water/feed ratio (and often related wet litter issues). What makes a vicious circle vicious is the poor response of acting on one of the steps of the circle only. A combined action focusing on breaking the circle at the four steps simultaneously is more successful. A good example is the use of antibiotics (such as AGP or therapeutics) to break the second step. If continuously administered, antibiotics do control BE (in feed AGP) but the moment they are withdrawn, the circle regains immediately traction (therapeutics).
Figure 2- Bacterial Enteritis Vicious Circle (De Gussem, 2010)
III. ROLE OF INSTIGATORS – FROM LOW HANGING FRUIT ON COCCIDIOSIS TO NOVEL INSIGHTS ON VIRAL CHALLENGES
Traditionally, coccidiosis has been the low hanging fruit on preventing BE issues. Eimeria spp. infections in broilers have been very well studied and there are a number of novel strategies that have been developed over the last decades in order to cope with the direct and indirect consequences of broiler coccidiosis. In an earlier review (De Gussem, 2007), 0.1€ per 2.5 kg broiler has been put forward as an average loss from subclinical coccidiosis (due to higher FCR and lower ADG). Recently, Blake et al. (2020) calculated an even higher amount but it is clear that together with BE (also estimated at an average 0.1€ cost per bird), coccidiosis is the most costly gut health entity in global poultry production and together these two disease entities are estimated for a loss of over 10B€ yearly.
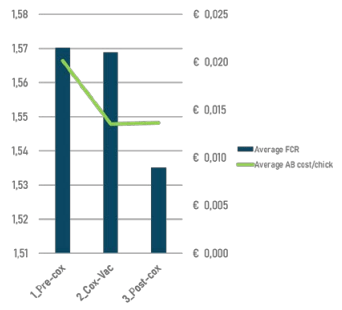
Novel strategies include restoration of efficacy of in-feed anticoccidials by resting them and rotating to other product and product classes, but also by rotating with live vaccines (consisting of sensitive strains) that have the advantage of accelerating the restoration of sensitivity. The effects on performance but also on BE related treatments have been demonstrated in several trials (Figure 3), confirming the intimate relation between the two diseases.
Figure 3 - Impact of live coccidiosis vaccination campaign on performance and Bacterial Enteritis antibiotic use based on data of 113 flocks of 9 different farms.
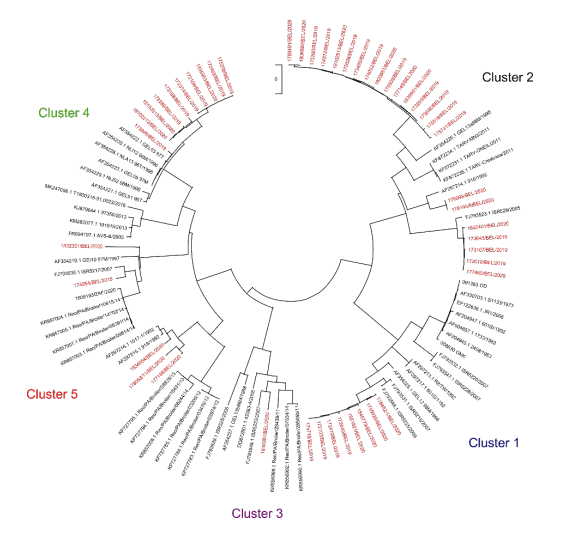
On the viral side, novel insights are helping to develop new strategies to cope with BE without having to give on performance. In a recent survey (not published) in Belgium, the majority of strains isolated from broiler and breeder farms are categorised after sequencing into Cluster 2 and Cluster 4. Surprisingly the third most abundant cluster retrieved was Cluster 1, although breeder flocks in Belgium are most commonly vaccinated with Cluster 1 vaccines. These findings have led to the insight that vaccines that consist of an older strain isolated in 1973 most probably have led to escape not only of genetically distant strains from other clusters (which would be expected) but also of Cluster 1 strains that are genetically different from the vaccine strains. This kind of antigenic drifting is now leading to redesign of vaccination programs to include also autogenous vaccine strains that are tailor made to the farms concerned. For this strategy, the most pathogenic strains from these farms, as defined in virulence typing work, are included.
Figure 4 - Cluster analyses of strains isolated in Belgium of ARV in 2019 and 2020 indicated in red.
IV. STRATEGIC USE OF ADDITIVES AND MEDICATION: TRADITIONAL VERSUS ALTERNATIVES?
The choice of best (so called traditional and alternative) solutions should be tailor-made for each operation, looking at the 4 steps of the vicious circle and what contributing factors are causing damage to intestinal health. Solutions work on different parts of the BE circle. Some products have antagonistic, some additive and some have a synergistic effect. Some products will go through the drinking water, some will go through feed or can be sprayed on the chicks or even in the environment.
Examples of traditional additives are antimicrobial growth promoters, anticoccidials, feed enzymes, antibiotics, coccidiosis vaccines, acids used as feed preservative, mycotoxin binders. Examples of alternatives are acids used to steer gut microbiota, probiotics, etheric oils, bacteriophages, beta-glucans. In fact, the differentiation is very artificial, and the ones that are alternative now will be standard in few years’ time. Therefore, they should all just be categorised as gut health support tools.
The objective of the poultry industry is to define what tools are relevant in terms of efficacy and cost for your operation with best return on investment (ROI). It all starts with understanding where the BE circle gets fuelled. Usually, it will be a complex combination of challenges, but with a good starting diagnosis of flocks one will understand where is the low hanging fruit. Coccidiosis for instance is very often the main primary instigator of the BE circle. The levels at a given farm can be very different from neighbouring farms and understanding this can help to define what impact improved coccidiosis control can have on BE or, if well controlled, if resources can be shifted to coping with other challenges such as mycotoxins, microbiota management, inflammation at level of gut, supporting poor absorption due to BE or dealing with litter quality issues.
It is important to understand that, for most production units, if coccidiosis levels are higher than average, it is very unlikely the best return on investment will yield from adding a probiotic in drinking water or working on the 3rd step of the BE circle, by adding antiinflammatory capacity. Changing the anticoccidial program will commonly give the best results as this will have a direct impact on performance, next to indirectly reducing BE.
So, it all starts with making a good diagnosis of what is going on by using scoring methods to define the levels of gut health challenge; both coccidiosis and BE scores. Next will be listing all what is being done already to support gut health and estimate a cost per kg bird produced for each of them. Other diagnostics typically will involve assessing mycotoxin and viral challenges, next to a nutritional evaluation to assess the complexity (crude protein digestibility, fat quality, anti-nutritional factors, structure, non-starch polysaccharides).
This data is then to be analysed to understand where breaking the BE circle can be most successful and where it is unlikely to be broken, and importantly at what cost. Once this has been defined, choosing gut health support tools becomes a relatively easier task, with usually huge impact on performance and ease of growing birds and very importantly with lower cost of gut health support tools but also lower FCR and higher ADG.
In order to develop and evaluate strategies in a controlled environment, (subclinical) NE models have been often used, but they don’t reflect the right level of challenge occurring in the field as subclinical NE is a rare finding in broiler operations, thus often not returning the right dose levels, or dose regimes of additives to cope with BE. Recently, a model to investigate BE has been developed. This model is combining feed complexity, with a mild coccidiosis challenge, a broad-spectrum antibacterial treatment and a bacterial cocktail orally administered. It simulates a mild BE (score 3/10) with a moderate performance impact, that is reflecting the BE status of the majority of operations worldwide. Using a model can accelerate and support findings from in vitro and field trials.
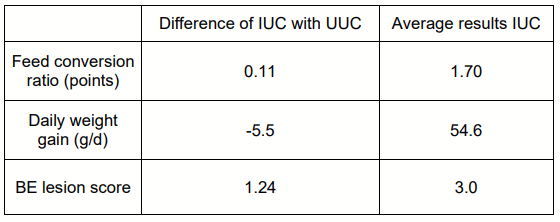
Table 1 - BE in vivo model. Average result of 12 trials (Poulpharm, 2020).
V. CONCLUSIONS
In poultry production, intestinal health is capital for performance. Since the ban of antimicrobial growth promoters more than 10 years ago in EU, research has demonstrated the importance for general health of the intestinal microbial community and the complex interaction with the host. Intestinal health can be achieved by improved feed composition, medication and additives to maintain a rich and diverse microbial community, and to control the host reaction through dietary immunomodulation. Bacterial Enteritis, a typical vicious circle pathology involving a dysbiosis and macroscopic visible signs of inflammation, has been recognised as an important gut health issue, next to coccidiosis. The complexity of BE ant the vicious circle characteristics requires a holistic approach in diagnosis, prevention and treatment of the disease.
Novel insight in coccidiosis control, viral challenges and use of dedicated in vivo models will further boost the understanding and control of BE, allowing the broiler industry to continue to produce birds with faster growth rates thus lower FCR in a sustainable way.
Presented at the 32th Annual Australian Poultry Science Symposium 2021. For information on the next edition, click here. 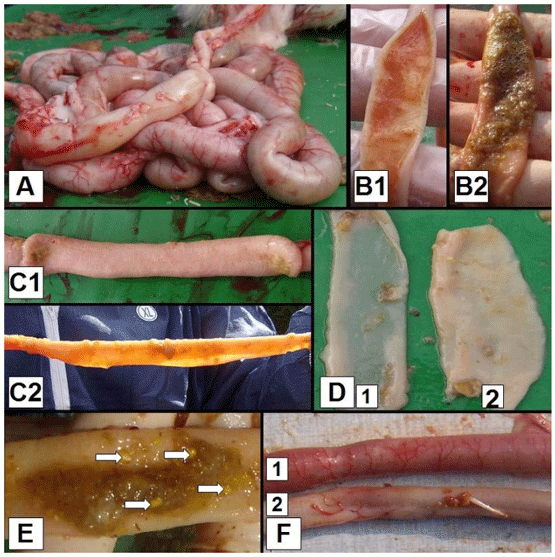










.jpg&w=3840&q=75)