Introduction
Increasing scientific awareness of the role of some intestinal bacteria in promoting health and improving production has enhanced the use of probiotic bacteria as active functional ingredients in animal and human nutrition. Delivery of probiotic strains of lactic acid bacteria (LAB)to poultry may be mediated by addition to either the water or the feed. Added benefit may be obtained if the feed is allowed to ferment to produce a feed containing at least 150 mmol lactic acid and a low pH, as this has been shown to reduce contamination of feed by enteropathogens such as Salmonellae (Heres et al 2003). Edens (2003) suggested selection criteria for potential probiotics in order to achieve well established and positive probiotic effects, besafe for the host and for the strains to be viable as well as metabolically active within the gastrointestinal tract (GI). The objective of this study was to isolate, characterise and select beneficial lactobacillus strains in an attempt to predict candidates that could be used in vivo as chicken probiotic adjuncts. In this study, a total of 53 lactic acid bacteria (LAB) isolated from the contents of the crop, caecum and small intestine, and from the mucosa of the crop, jejunum and ileum of three organically farmed chickens were examined for their probiotic properties.. In vitro methods were used for screening potential probiotic strains using a dynamic model that mimics in vivo GI conditions as closely as possible.
Materials and methods
All 53 LAB were assessed for autoaggregation according to the method of Kmet and Lucchini (1999). The reaction times giving the degree of aggregation intensity were, rapid: 15min, normal: 15-30 min and slow: 30-60min. Coaggregation with S.enteritidis 5188, S.enteritidis (PHL Exeter) I.Ap.29 (chicken), S.typhimurium AFCC 14028, E.coli and Clostridiun perfringens NCIB 8693 was assessed according to the method of Drago et al. (1997). Suspensions were prepared for observation by scanning electron microscope (JEOL 5600 Low Vacuum SEM). Rapidly aggregating strains and strains that showed coaggregation with Salmonella spp were selected and identified using API CHL kit (BioMéreux, UK) and apiweb® (BioMéreux, UK), the appropriate identification software. Antagonistic activity of the selected lactobacilli towards S. enteritidis (5188), S.enteritidis of chicken origin, S.typhimurium, E.coli and Cl.perfringens was conducted according to the agar spot test described by Jin et al. (1996). Lactobacilli were also tested for their ability to survive under the conditions prevailing in the gut of the chicken.Ten mls of an overnight culture were sprayed over 100g of chicken feed, and incubated in room temperature for 30mins. Ten g of the spayed feed were then added to a flask filled with 70mls of distilled water and heated in a water bath to 41.4oC (chickens body temperature) The pH was adjusted to pH 4.4 - 4.5 for 45 mins, then to pH 2.6 for 45 mins and finally to pH 6.2 for 90 mins to simulate conditions in the crop, gizzard, ileum, respectively. The number for viable LAB was enumerated by serial dilution, plating onto MRS agar and incubating for 48h at 37oC. The mucus binding assay was conducted according the methodology described by Styraik et al (1999a), using mucin from porcine stomach Type II solution, (Sigma, UK). Lactobacilli were classified as strongly adherent (A550nm> 0.3), weakly adherent (<0.1<A550nm> 0.3), or nonadherent <0.1<A550nm. The selected Lactobacillus isolates were assessed on their ability to adhere to the chicken gut epithelial cells in vitro, according to the methodology described first by (Fuller, 1973) and also used later by (Garriga et al., 1998). Scanning electron microscopy pictures of each lactobacillus- epithelial cell suspension were taken to confirm the adhesion of the lactic acid bacteria to intestinal epithelial cells. The methodology used for studying the hydrophobic interactions of cells is based on the well established MATH (Microbial adhesion to hydrocarbons) assay (Rosenberg et al., 1980), following the modifications of (Gusils et al., 2002). The survivability of L.plantarum in both distilled and hard water (0.05 mg l-1 of sodium hypochlorite solution 12% w/v and 0.04 mg l-1 of calcium carbonate in distil water) at two different temperatures 20ºC and 35oC was also determined.. L.plantarum and L.salivarius were also tested for their potential ability to ferment the cereal component (wheat or barley) of chicken feed. Samples were analysed for production of lactic by HPLC according to the method of (Niven, Beal et al. 2004). The data was subjected to ANOVA using the General Linear Model (GLM) procedure of MINITAB v.14.0. Death rate of LAB at different temperature and different time interval was converted to log10 before applying to statistical analysis.
Results
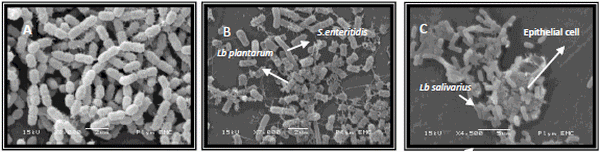
From 53 LAB that were tested for their capacity to aggregate, 20 were non-aggregative. Eleven bacteria showed a rapid autoaggregation, within 15minutes. The 23 LAB that showed normal and rapid aggregative activity were further tested for their ability to coaggregate with several enetropathogens. One LAB strain showed maximum aggregation, two showed marked aggregation, six showed good aggregation, nine partial aggregation and three showed no or almost no aggregation. Results were confirmed by scanning electron (figure I). Those lactobacilli that showed maximum and marked aggregation were selected to be identified by the API identification kit. The results are shown in table I.
Table I. Origin, identification, autoagreggation of lactobacilli, and their coaggregation with Salmonela enteritidis 5188, Salmonella enteritidis (PH Exeter) I.Ap.29 (chicken), Salmonella typhimurium AFCC 14028, Escherichia coli sp. and Clostridiun perfringens NCIB 8693
 a
a The score is based upon (Kmet and Lucchini, 1999) methodology.
b The score is based upon (Drago et al., 1997) methodology
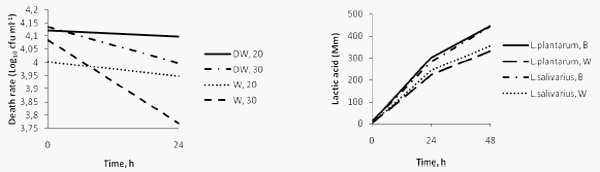
All of the LAB tested survived transit through the stimulated GI tract and were found to be equally resistant to the acidic environment (P>0.05). Eleven LAB were tested for adherence to porcine mucus8 found to be non adherent to porcine mucus and two Lb plantarum and Lb fermentum were found to be weakly adherent. All the lactobacilli tested showed that are able to inhibit the growth of S enteritidis (5188), S.enteritidis of chicken origin, S.typhimurium, E.coli and Cl.perfringens to varying degrees. The results are shown in table II. Lb plantarum and Lb salivarius showed stronger antibacterial properties against the pathogens than Lb fermentum and Leuconostoc lactis. The percentage of the hydrophobicity of Lb plantarum and Lb salivarius in hexadecane, toluene and xylene is shown on table III. Lb.plantarum remained alive after 24h in the water, though the death rate was higher at 30oC than at 20oC (figure II). Figure III presents the production of lactic acid after 24 and 48h fermentation at 30oC of barley and wheat with Lb.plantarum and Lb. salivarius.
Table II. Antagonistic activity of Lactobacillus spp. isolated form chickens against several enteropathogens. (Each value is the mean of the triplicate of each sample).
Figure I: A. Rapidly aggregating Lactobacillus sp. B. Lb plantarum showed maximum aggregation with S.enetritidis. C. Adherence of Lb salivarius to chicken gut epithelial cell.
Table III: Hydrophobicity of Lb plantarum and Lb salivarius in different solvents (Each value is the mean of the triplicate of each sample, Lb acidophilus was used as negative control).
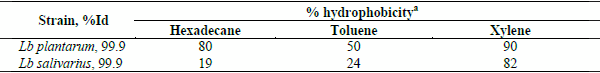 a
a % hydrophobicity = (OD600 before mixing - OD600 after mixing) x 100 / OD600 before mixing
Figure II. Death rate of Lb plantarum in distilled water (DW) and hard water (W) at 30oC and 20oC. (Each value is the mean of the triplicate of each sample). Figure III. Production of lactic acid after 24 and 48h fermentation at 30oC of Barley (B) and Wheat (W) with Lb plantarum and Lb salivarius. (Each value is the mean of the triplicate of each sample).
Conclusion
The probiotic properties of lactic acid bacteria have been widely studied, demonstrating that their capability of adhering to mucus to epithelial cells is one of the potential mechanisms of providing a competitive advantage in the intestinal microbiota (Ghadban 2002) and consequently inhibiting the in vitro growth of enteropathogens. Autoaggregation of lactic acid bacteria may be necessary for adhesion to intestinal epithelial cells and with the addition of their potential coaggegation ability may form a barrier that prevents colonization of pathogenic microorganisms (Kos, Suskovic et al. 2003). Both Lb plantarum and Lb salivarius showed high values of hydrophobicity, which according to Gusils (1999) indicates a strong ability of the bacteria to adhere to epithelial cells.
The water type does not seem to affect the survival of Lb plantarum as the strain managed to survive up to 24 hr in both hard and distilled water. The insignificant death rate does not greatly affect the number of Lb. plantarum after 24 hr, which might suggests that that environmental temperature does not influence the survival of LAB in the water.
According to Heres et al (2003) the high numbers of lactic acid bacteria and the high concentration of lactic acid in the fermented feed could make chickens less susceptible to Salmonella infections.
Both Lb plantarum and Lb salivarius strains that have tested found to be capable to ferment chicken feeds, producing about 380mmol lactic acid and lowering the pH of the feed to 3.6 - 3.7 after 24h incubation at 30oC.
The results indicate that two strains of lactobacilli, Lb plantarum and Lb salivarius, isolated from the natural gut microflora of poultry, exhibited strong potential as probiotic adjuncts and could perform effectively within the GI tract. However, given the complexity of the chicken GI tract, the proof of efficacy of the two probiotic bacteria in broilers will require in vivo studies.
Abstract presented at the 1st Mediterranean Summit of World's Poultry Science Association (WPSA).
References
Boris, S., Suarez, J. E., Vazquez, F. & Barbes, C. (1998) Infection and Immunity, 66, 1985-1989.
Drago, D., Gismondo, M. R., Lombardi, A., De Haen, C. & Gozzini, L. (1997) Fems Microbiology Letters, 153, 455-463.
Edens, F. W. Brazilian Journal of Poultry Science (2003) 5, 75-97.
Fuller, R. (1973) Journal of Applied Bacteriology 36, 131-139.
Ghadban, G. S. (2002) Archiv Fur Geflugelkunde, 66, 49-58.
Gusils, C., Perez, A. C., Gonzales, S. & Oliver, G. (1999) Journal of Food Protection, 62, 252-256.
Gusils, C., Bujazha, M., Gonzales, S. (2002) Intercencia, 27, 409-413.
Jin, L.Z., Ho, Y.W., Abdullah N., Ali M.A., Jalaludin S (1996) Letters of Applied Microbiology, 23, 67-71.
Heres, L., Engel, B., van Knapen, F., de Jong, M.C.M., Wagenaar, J.A. & Urlings H.A.P. (2003) Poultry Science, 82, 603-611.
Kmet, V. & Lucchini, F. (1999) Journal of Veterinary Medicine Series B-Infectious Diseases and Veterinary Public Health, 46, 683-687.
Kos, B., Suskovic, J., Vukovic, S., Simpraga, M., Frece, J. & Matosic, S. (2003) Journal of Applied Microbiology, 94, 981-987.
Niven, S. J., J. D. Beal, et al. (2004) Animal Feed Science and Technology 117, 339-345.
Revolledo, L., Ferreira, A. J. P. & Mead, G. C. (2006) Journal of Applied Poultry Research 15, 341-351.
Rosenberg, M., Gutnick, D., Rosenberg, E. (1980) FEMS Microbiology Letters, 9, 29-33Styriak, I., Demecková, V., Zatkovic, B. & Kmet, V. (2001) Veterinary Medicine-Chech, 46, 89-94.












.jpg&w=3840&q=75)