Introduction
Transmission of APEC (Avian Pathogenic Escherichia coli) strains through contamination of incubable eggs or embryo infection has been described (Barnes et to the., 2003). It can happen that, although few chicken are already infected at hatching, the dissemination of the strains takes place through horizontal transmission at the time of hatching, thus infecting a large numbers of chicken from the same or other breeding batches (Dhillon & Jack, 1996). These APEC strains can lead to local infections, such as Omphalitis and cellulitis infection or to systemic infections; the major pathologies being perihepatitis, pericarditis, aerosaculitis, pneumonia, and cases of synovitis and osteomyelitis.
Bacterial condronecrosis with osteomyelitis results in lameness that causes delay in growth, poor chicken quality and slaughterhouse seizures, causing major economic losses for the poultry industry. Although other bacteria, mainly Staphylococcus aureus, produce a similar clinical picture, Escherichia coli has also been described as a major cause of these problems (McNamee & Smith, 2000).
The objective of this study was to determine if Escherichia coli was the infectious agent implicated in the process, study the antibiotic sensitivity profile of isolated agents and investigate the circulating clones, as well as the possible origin and dissemination thereof.
Materials & Methods
Several batches of broilers began showing lameness from the second week of life; these batches, in general, came from three breeding stock farms (A, B and C). 60 newly hatched chicken were analyzed, 20 from breeding farm A, 20 from breeding farm B and 20 from breeding farm C. 10 chicken from each batch were analysed prior to vaccination (batch A1, batch B1 and batch C1) and the other 10 were analyzed alter respective vaccinationed, together with gentamicin (batch A2, batch B2 and batch C2).
The samples that were analyzed were yolk sac and femur bone marrow, following the method described by McNamee et al. (1998). Samples were cultured in blood agar, MacConkey agar, salt manitol, Enterococcosel agar and in the case of bones, in brain-heart-infusion broth. In the latter case, after 24 hours of enrichment at 37° C, the broth media were seeded in the solid media listed above.
The Escherichia coli isolates obtained were examined to determine their sensitivity to different antimicrobials using the disk diffusion method.. Furthermore, they were characterized by pulsating field electrophoresis (PFGE), following the Protocol described by Pulsenet (Ribot et al., 2006).
Results and Discussion
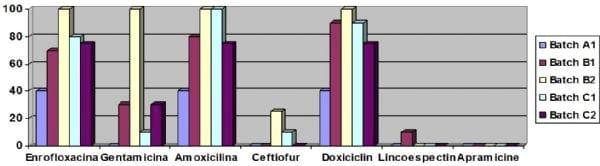
The results of the impact of Escherichia coli in different batches and samples are listed in table 1. The results are summarized in Figure 1.
Table 1: Incidence of e. coli in the batches of chicken in relation to the analyzed samples.
Figure 1: Percentage of resistance to different antibicrobial drugs by batch.
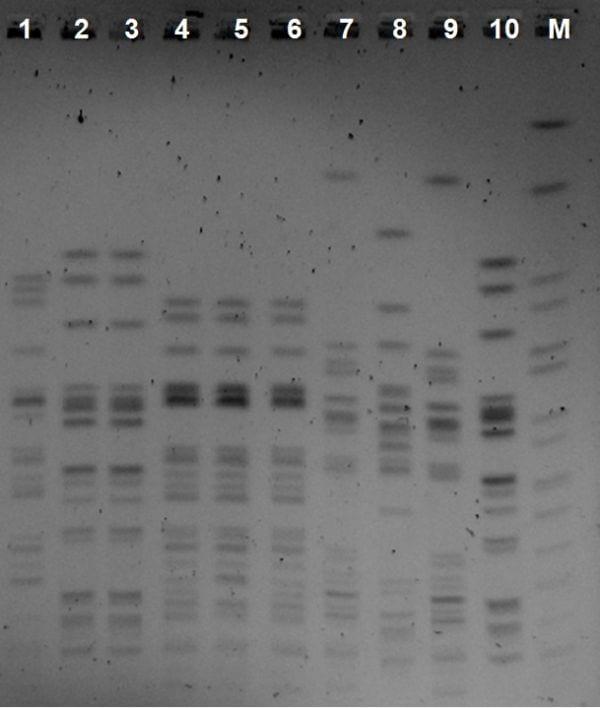
The fact that 4 gentamicin-sensitive strains were isolated in batch C2 batch could be explained by failure in the administration of the vaccine, since 2 of them come from the Yone sac and bone of the same chicken. A total of 12 pulse types were obtained using the pulse field technique electrophoresis. Two of them were the majority (Figure 2), one of them was detected in bone and yone sac of the three previously vaccinated Chicken and one more, resistant to gentamicin, only in bone of batches B2 and C2, i.e., after vaccination.
Figure 2: Pulse field electrophoresis image
Lanes 2, 3 and 10 lanes correspond to the gentamicin-resistant clone and lanes 4, 5 and 6 correspond to the bone and yolk sac clone isolated from from the three batches of breeding chicken. The other pulse types relate to non-majority clones. Lane M: Molecular weight marker.
The first noteworthy point is the high percentage of Escherichia coli isoalates from unvaccinated chicken yolk sac bone, which has been observed in other integrations with high mortality issues high in first weeks of life (García Peña et al., 2009a). This fact, together with the isolation of strains of a same pulse type in chicken coming from different breeding farms, suggests that the entry of these strains in the production chain took place via contaminated eggs and subsequent dissemination at the time of hatching (García Peña et al., 2009b). Thus, the vertical transmission of strains of the same Esherichia coli clone, resistant to fluoroquinolone has been described. Furthermore, this clone produced low embryonic mortality, although later on, it gave rise to colibacillosis issues in the farm (Petersen et al., 2006). It would be necessary to analyze cloacal swabs of the breeding stock of three farms in order to confirm if there is vertical transmission and determine the origin of the strains.
Osteomyelitis by Escherichia coli usually occurs after a generalized infection (Hofacre, 2003). However, the clinic picture observed in the broilers consists of the appearance of lameness from the second week of life, without observing problems at the start of the chicken or other pathologies, such as pericarditis, perihepatitis, etc. This could be due to these strains a particular tropism for the bone marrow, which would explain the higher percentage of isolates in bone vs. yolk sac in chicken without vaccination. Also, chicken are treated with an antibiotic when symptoms begin, thus removing the widespread infection, but the antibiotic probably does not reach the proper concentration or is not given enough time to eliminate the bone tissue infection.
Another important aspect is the high percentage of multi-resistant strains. Thus, 30% of the strains are resistant to enrofloxacin, amoxicillin and doxycycline and another 33 per cent had the same profile of resistance but with the addition of gentamicin. The antibiotics showing lower percentage of resistance and, therefore, the most advisable for treatment were the lincoespectinomicine and the apramicine.
Conclusions
The isolation of strains of Escherichia coli belonging to the same pulse type from newly hatched chicken implies that there is a common source of infection, which would be contaminated eggs from some of the breeding farms. In the incubator, there would be contamination to chicks of other batches, mainly after hatching.
In this study, a gentamicin-resistant clone was selected, showing a particular tropism for bone marrow , causing an outbreak of difficult-to-treat osteomielitis, both because of the high percentage of strains resistant to several antibiotics widely used in poultry, as well as because of the difficulty that all antibiotics have in reaching bone tissue.
As a consequence, the indiscriminate use of antibiotics leads to a selection of multiresistant strains, making it very difficult to treat colibacillosis outbreaks and, also, posing a risk to public health.
Bibliography
Barnes HJ, Vaillancourt JP, Gross WB. 2003. Colibacillosis. pp. 631-657. In Diseases of poultry. Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR (Eds). Blackwell Publishing, Ames, Iowa (USA).
Dhillon AS & Jack OK. 1996. Two outbreaks of colibacillosis in commercial caged layers. Avian Dis. 40:742-746.
García Peña FJ, Abad Moreno JC, Serrano T, Jimenez C, Herrero A. 2009a. Estudio de un caso de colibacilosis en una integración avícola. Simposium WPSA/AECA. Zaragoza (España).
García Peña FJ, Abad Moreno JC, Herrera S, Echeita A, Serrano T, Jimenez C, Herrero A. 2009b. Investigación de un caso de colibacilosis en una integración de pollos de engorde. XVI Congress of the World Veterinary Poultry Association. Marrakech (Marruecos).
Hofacre CH. 2003. Colibacilosis aviar: patogénesis y epidemiología. Mundo Ganadero: 48-49.
McNamee PT & Smith JA. 2000. Bacterial chondronecrosis with osteomyelitis ("femoral head necrosis") of broiler chickens: a review. Avian Pathol. 29:253-270.
McNamee PT, McCullagh JJ, Thorp BH, Ball HJ, Graham D, McCullough SJ, McConaghy D, Smyth JA. 1998. Study of leg weakness in two commercial broiler flocks. Vet. Rec. 143(5):131-135.
Petersen A, Christensen JP, Kuhnert P, Bisgaard M, Olsen JE. 2006. Vertical transmission of a fluoroquinolone-resistant Escherichia coli within an integrated broiler operation. Vet. Microbiol. 116:120-128.
Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ. 2006. Standardization of Pulsed-Field Gel Electrophoresis Protocols for the Subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodbourne Pathog. Dis. 3:59-67.








.jpg&w=3840&q=75)