Improving phosphorus availability in broiler diets based on wheat-soybean meal using microbial phytase produced in solid state fermentation
For the phytate-P to be utilised by monogastric animals, the phytate must be hydrolysed into inorganic phosphorus. The dephosphorylation of phytic acid requires phytases, a class of enzymes that catalyse the removal of the six inorganic P molecules from phytic acid in a stepwise manner. During the past decade, advances in biotechnology and fermentation technology have resulted in the large-scale production of microbial phytases capable of hydrolyzing phytic acid and releasing phytate-bound P. Two distinct phytase products are available in the market – one derived from submerged liquid fermentation that uses genetically manipulated organisms to achieve maximum enzyme production and the other based on solid state fermentation that uses unmodified organisms for enzyme production. Allzyme Phytase represents the latter group and is produced by growing Aspergillus niger on water-insoluble substrates in the presence of minimal free-flowing water. The aims of the present study were to study the influence of Allzyme Phytase on performance, phytate phosphorus release and retention and excretion of nutrients in wheat-soy diets for broiler chickens.
Materials and methods
Day-old broiler (Ross) chicks, females and males, were obtained from a commercial hatchery and randomly assigned to 80 pens (8 birds/pen) in 3- tier electrically heated battery brooders. The brooders were housed in an environmentally controlled room with 24-hour fluorescent lighting. Within each sex (female or male), the eight dietary treatments were randomly assigned to five pens of eight chicks each. The birds were transferred to colony cages in an environmentally controlled room on day 14. Room temperature was maintained at 32 ± 1°C during the first week and gradually decreased to 24°C by the end of the third week.
Table 1.Percentage composition and calculated analysis of diets for broiler starters.
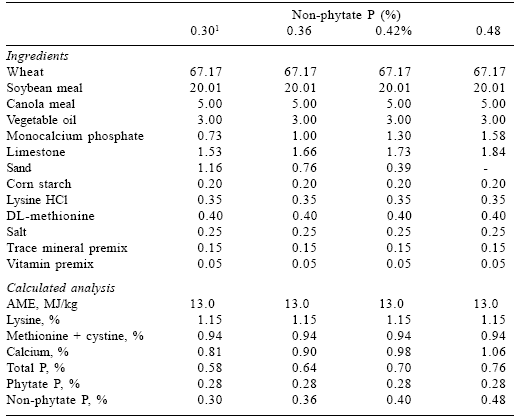
10, 500, 1000, 1500 and 2000 U of Allzyme Phytase were added to the diet containing 0.30% non-phytate P. Allzyme Phytase was assayed to contain 1052 PU/g. One unit of phytase (PU) is defined as the quantity of enzyme that releases 1 μmol of inorganic P/min from 0.00015 mol/L sodium phytate at pH 5.5 at 37 °C .
The basal diet was based on wheat, soybean meal and canola meal. Two sets of basal diets were used: one for broiler starters (1-21 days) and the other for broiler finishers (22-42 days). The basal diet for broiler starters was formulated to meet or exceed recommended specifications for all nutrients except phosphorus and calcium (Table 1). The non-phytate P level was maintained at 0.30%. This level was selected to maintain dietary available P below current NRC (1994) recommendations and to ensure responses with inorganic phosphate and phytase additions. This basal diet was supplemented with monocalcium phosphate to provide three levels of non phytate P (0.36, 0.42 and 0.48%) or with four levels of Allzyme Phytase (500-2000 PU/kg diet). One unit of phytase (PU) is defined as the quantity of enzyme that releases 1 μmol of inorganic P/min from 0.00015 mol/L sodium phytate at pH 5.5 at 37°C. Phytase was added in place of corn starch and sand was used to replace monocalcium phosphate and limestone. The calcium (Ca):total P ratio was maintained at 1.4:1 in all diets. The enzyme was first mixed into the premix and then into the diet. The diets were cold-pelleted (65°C) using a 3-mm die. The wheat used in the study was first steam-pelleted at 90°C to reduce the intrinsic phytase activity and then ground prior to mixing into feed.
For the broiler finisher phase, the basal diet contained 0.20% non-phytate P (Table 2) and was supplemented with monocalcium phosphate to provide three levels of non-phytate P (0.26, 0.32 and 0.38%) or with four levels of Allzyme Phytase (500-2000 PU/kg diet). The Ca:total P ratio was maintained at 1.4:1. Titanium oxide (0.05%) was included in all finisher diets as a dietary marker.
Table 2.Percentage composition and calculated analysis of diets for broiler finisher diets.
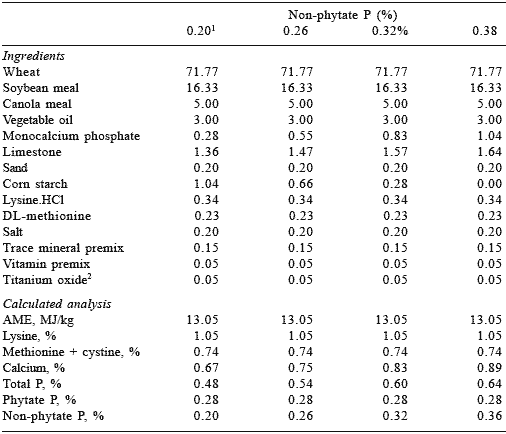
1 0, 500, 1000, 1500 and 2000 U of Allzyme phytase were added to the diet containing 0.30% non-phytate P. Allzyme Phytase was assayed to contain 1052 PU/g.
2Titanium oxide was incorporated as an indigestible dietary marker.
Body weights and feed intake were recorded on a pen basis at weekly intervals. Feed was offered ad libitum and water was freely available during the 42-day trial period. Mortality and morbidity were observed and recorded daily. Any bird that died was weighed and the weight was used to adjust feed conversion. Feed conversion ratios were calculated by dividing total feed intake by weight of live plus dead birds. During the third week (day 18-21) and sixth week (day 39-42), total collection of excreta was carried out for the determination of apparent metabolizable energy and nutrient retention/excretion. Feed intake and excreta output were measured quantitatively per pen over the four consecutive days. On day 42, all surviving birds were euthanized and digesta contents from the lower half of the ileum collected for ileal digestibility determinations. The nutrient retention and digestibility data, however, will not be presented in this paper. Toe samples were obtained for toe ash measurements by severing the middle toe through the joint between the 2nd and 3rd tarsal bones from the distal end. Toe ash has been shown to be a good indicator of P status and is very accurate in determining P availability for poultry (Potter, 1988).
Pen means served as the experimental unit for statistical analysis. Mortality data were transformed to arc sine prior to analysis. The data were analyzed by the General Linear Models procedure of the SAS® (SAS Institute, 1990) with pen mean as the experimental unit. Since sex by treatment interaction was not of interest, data from male and female broilers were analyzed separately. Linear and quadratic effects of inorganic P from monocalcium phosphate (Diets 1 to 4) and supplemental phytase (Diet 1 and Diets 5 to 8) on gain, feed intake, feed/gain and toe ash were tested using orthogonal polynomials.
Results
Mortality to 42 days of age in this study was higher than expected (5.6%). Most of the deaths occurred between days 15 and 18 and appear to have been exacerbated by the stress of moving the birds from the brooder to colony cages on day 14. Mortality, however, was not influenced (P > 0.10) by dietary levels of non-phytate P or phytase.
RESPONSES DURING THE BROILER STARTER PHASE
(1-21 DAYS OF AGE) Dietary treatments significantly influenced the performance of male and female broilers (Tables 3 and 4). In males, increasing dietary non-phytate P levels improved (P<0.10 to 0.001) weight gain, feed intake and feed/gain, but an unexplainable quadratic effect (P<0.10 to 0.01) was observed at 0.48% non-phytate P. Weight gain and feed/gain were improved quadratically (P<0.01) and feed intake linearly (P<0.05) with increasing dietary additions of phytase. In females, weight gain and feed/gain improved linearly (P<0.05 to 0.001) as the level of non-phytate P in the diet increased. Dietary non phytate P had no effect on feed intake. Increasing additions of phytase quadratically (P<0.05 to 0.001) improved weight gain and feed/gain, and linearly (P<0.001) increased feed intake.
Table 3.Effect of dietary P and Allzyme Phytase levels on performance parameters of male broilers (days 1-21).
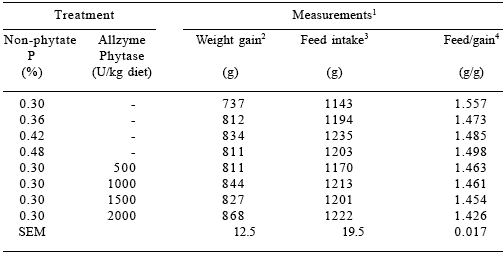
1Each mean represents 5 pens (8 birds per pen). Feed:gain values corrected for mortality.
2Phosphorus effect (linear, P<0.001; quadratic, P<0.01). Phytase effect (linear, P<0.001; quadratic, P<0.01).
3 Phosphorus effect (linear, P<0.05; quadratic, P<0.10). Phytase effect (linear, P<0.05).
4Phosphorus effect (linear, P<0.10; quadratic, P<0.05). Phytase effect (linear, P<0.001; quadratic, P<0.01).
Table 4.Effect of dietary P and Allzyme Phytase levels on performance parameters of female broilers (days 1-21).
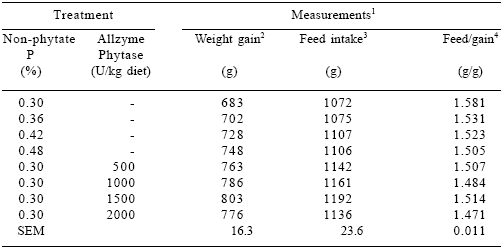
1Each mean represents 5 pens (8 birds per pen). Feed:gain values corrected for mortality.
2Phosphorus effect (linear, P<0.05). Phytase effect (linear, P<0.001; quadratic, P<0.05).
3Phytase effect (linear, P<0.001).
4Phosphorus effect (linear, P<0.001). Phytase effect (linear, P<0.001; quadratic, P<0.001).
In both sexes, the performance of birds fed 0.30% non-phytate P diets with 1000 U phytase/kg were similar (P>0.10) to those fed the 0.48% non-phytate P diet.
RESPONSES DURING THE BROILER FINISHER PHASE
(22-42 DAYS OF AGE) In males, increasing dietary non-phytate P levels quadratically (P<0.05) increased feed intake and tended (P=0.11) to improve weight gain and feed/ gain (Table 5). Phytase addition had no effect on feed intake, but caused linear (P<0.001) increases in weight gains and quadratic responses in feed/ gain. In females, dietary non-phytate P linearly (P<0.05) improved weight gain and tended (P=0.11) to improve feed/gain (Table 6). Increasing additions of phytase quadratically improved weight gain (P<0.10) and feed/gain (P<0.001). Feed intake was unaffected by phytase levels.
Table 5.Effect of dietary P and Allzyme Phytase levels on performance parameters of male broilers (days 22-42).
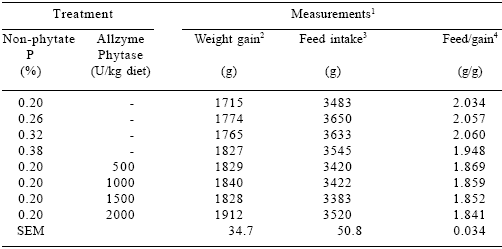
1Each mean represents 5 pens (8 birds per pen). Feed:gain values corrected for mortality.
2Phosphorus effect (linear, P=0.11). Phytase effect (linear, P<0.001).
3Phosphorus effect (quadratic, P<0.05).
4Phosphorus effect (quadratic, P=0.11). Phytase effect (linear, P<0.001; quadratic, P<0.05).
In both sexes, performance of birds fed diets containing 0.20% non-phytate P with 1000 U phytase/kg were similar (P > 0.10) to those fed diets containing 0.38% non-phytate P.
OVERALL PERFORMANCE RESPONSE (1-42 DAYS OF AGE)
The influence of dietary treatments on the performance over the 42-day trial period in male and female broilers is summarized in Tables 7 and 8, respectively. In males, increasing non-phytate P levels had linear effects (P<0.05) on weight gain and feed/gain, and quadratic effects (P<0.05) on feed intake. Weight gain and feed intake improved quadratically (P<0.001) with increasing additions of phytase in the diet. Phytase had no effect on feed intake. In females, increasing non-phytate P levels linearly improved weight gain (P<0.01) and feed/gain (P<0.05). Feed intake was unaffected by dietary non-phytate P content. Increasing levels of phytase quadratically improved weight gain (P<0.05) and feed/gain (P<0.001), and linearly (P<0.05) increased feed intake.
In both sexes, the magnitude of improvement in response to added enzyme was greatest at 500 U/kg of diet and tended to plateau with further additions. It is also noteworthy that the gain and feed/gain of birds fed phytasesupplemented diets were numerically superior to those fed diets supplemented with monocalcium phosphate. The birds receiving the low-P diets with 1000 U phytase/kg had a similar weight gain and better (P<0.05) feed efficiency compared to those receiving P-adequate diets.
Table 6.Effect of dietary P and Allzyme Phytase levels on performance parameters of female broilers (days 22-42).
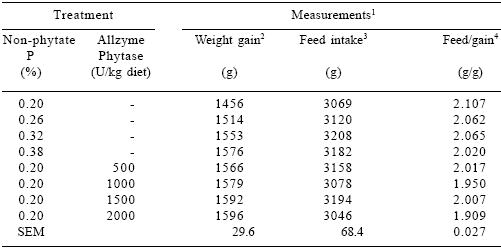
1Each mean represents 5 pens (8 birds per pen). Feed:gain values corrected for mortality.
2Phosphorus effect (linear, P<0.05). Phytase effect (linear, P<0.01; quadratic, P<0.10).
3Phosphorus effect (linear, P=0.11). Phytase effect (linear, P<0.001; quadratic, P<0.001).
EFFECTS OF DIETARY P AND ALLZYME PHYTASE ON TOE ASH CONTENT
No signs of leg weakness were observed in any of the diet groups during the trial. Increasing dietary levels of non-phytate P (linear, P<0.01; quadratic, P<0.10) and phytase (linear, P<0.001; quadratic, P<0.05) increased the ash percentage of toes in male broilers (Table 7). Toe ash content in female broilers increased as the level of non-phytate P (linear, P<0.001) and phytase (linear, P<0.001; quadratic, P<0.01) in the diet increased (Table 8).
Table 7.Effect of dietary P and Allzyme Phytase levels on performance parameters and toe ash content of male broilers (days 1-42).
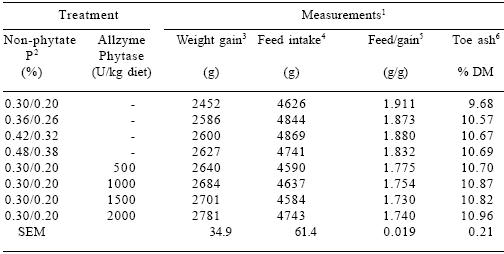
1Each mean represents 5 pens (8 birds per pen). Feed:gain values corrected for mortality.
2Non-phytate P sequence was used days 1-21 and 22-42, respectively.
3Phosphorus effect (linear, P<0.05). Phytase effect (linear, P<0.001; quadratic, P<0.001).
4Phosphorus effect (quadratic, P<0.05).
5Phosphorus effect (linear, P<0.05). Phytase effect (linear, P<0.001; quadratic, P<0.001).
6Phosphorus effect (linear, P<0.01; quadratic, P<0.10). Phytase effect (linear, P<0.001; quadratic, P<0.05).
Table 8.Effect of dietary P and Allzyme Phytase levels on performance parameters and toe ash content of female broilers1 (days 1-42).
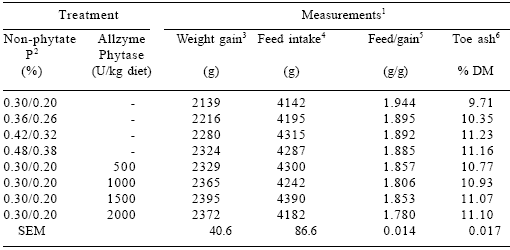
1Each mean represents 5 pens (8 birds per pen). Feed:gain values corrected for mortality.
2Non-phytate P sequence was used days 1-21 and 22-42, respectively.
3Phosphorus effect (linear, P<0.01). Phytase effect (linear, P<0.001; quadratic, P<0.05).
4Phytase effect (linear, P<0.05).
5Phosphorus effect (linear, P<0.05). Phytase effect (linear, P<0.001; quadratic, P<0.001).
6Phosphorus effect (linear, P<0.001). Phytase effect (linear, P<0.001; quadratic, P<0.01).
In both sexes, the magnitude of response was greatest at the first addition of non-phytate P or phytase, and tended to plateau with further additions. Interestingly, the toe ash contents of birds fed the low-P diet with 500 U phytase/kg of diet were comparable to those of birds fed adequate-P diets.
Discussion
The condition necessary for the study, ie., that growth and toe ash content in broilers fed the low-P diet would be low in order to respond to added inorganic P or phytase, was met. There were, however, no visible leg problems or excessive mortality in this treatment. Phytase supplementation of the low-P diet caused significant improvements in growth in both sexes. Increased weight gain was due both to an increase in feed intake and improvement in feed efficiency.
Interestingly, the responses in growth and toe ash beyond the first addition of inorganic P or the enzyme were minimal. The greatest responses were noted with the first addition and response reached a plateau with further additions. In males, over the 42-day trial period, compared to the low-P diet (containing 0.30% non-phytate P during the first 21 days and 0.20% nonphytate P during the last 21 days), addition of 500, 1000, 1500 and 2000 U phytase/kg diet increased weight gains by 7.7, 9.5, 10.2 and 13.4%; and lowered feed/gain by 7.1, 8.2, 9.5 and 8.9%, respectively. In females, the corresponding increments in weight gain were 8.9, 10.6, 12.0 and 10.9%, and reductions in feed/gain were 4.5, 7.1, 4.7 and 8.4%, respectively. It is noteworthy that the birds receiving the low-P diet with 1000 U phytase/kg had a similar weight gain and better feed efficiency compared to those receiving P-adequate diets.
PHOSPHORUS EQUIVALENCY
Equations based on performance responses obtained with supplemental nonphytate P and phytase have been previously used to calculate the equivalency value of phytase for inorganic P (Kornegay et al., 1996; Yi et al., 1996); and the estimation of a P replacement value for Allzyme Phytase was an aim of the current study. Linear and non-linear response functions of body weight gain, feed intake and feed efficiency that best fit the data were derived for inorganic P and phytase levels in both sexes and were used to determine the amount of P released. However, equations with moderate or high R2 values could not be consistently generated for feed intake and feed/ gain data although the probability values were significant.
Based on a limited number of equations with moderate to high R2 values for weight gain (data not presented), it was calculated that the amounts of non-phytate P equivalent to 1000 PU phytase/kg diet addition in male and female broilers during the broiler starter phase were 0.52 and 0.58 %, respectively. Therefore, released non-phytate P equaled P equivalency estimate minus non-phytate P in the basal diet (0.30%). The gain responses from 1000 PU phytase/kg of diet equal to 0.22 and 0.28% in males and females, respectively (Table 9). The corresponding non-phytate P replacement values during the broiler finisher phase were calculated to be 0.29 and 0.24%, respectively. Note that the phytase level of 1000 PU/kg diet was considered in these calculations, since this is the recommended inclusion rate of the enzyme.
Table 9.Phosphorus equivalency values for Allzyme Phytase based on linear and nonlinear functions generated from body weight gain during broiler phase (days 1-21).

*Phosphorus equivalency was calculated by subtracting the actual dietary non-phytate P content of the diet from the response relative to a known amount of inorganic (available) P.
The P equivalency values calculated above are clearly over-estimations. These calculations are based on the assumption (Kornegay et al., 1996; Yi et al., 1996) that the weight gain and feed/gain responses are due solely to increments in available P levels resulting from the addition of inorganic P or release of phytate-bound P with added phytase. While this assumption holds true for inorganic P additions, it is clear that factors unrelated to P availability are also responsible for the responses observed with added phytase. The finding that feed efficiency of birds fed phytase-supplemented diets when averaged across inclusion levels was superior to those fed diets supplemented with monocalcium phosphate throughout the 42-day trial period lends support to this view.
Among the possible reasons for the pronounced positive effect of Allzyme Phytase, two are especially worth noting. First, the Allzyme Phytase, being an enzyme preparation produced by solid-state fermentation, contains several side-enzyme activities besides phytase. In the present study, although it is routine to include exogenous xylanases in wheat-based diets, the use of xylanases was intentionally avoided. It is tempting therefore to speculate that the secondary enzymes may have played a part in improving nutrient availability from wheat. In addition, it has been recently suggested that added phytase may directly improve energy utilization in wheat (Ravindran et al., 1999). This thesis is based on the report by Frolich (1990) that phytate is an integral component of the cell wall matrix in wheat. Microbial phytase may therefore be expected to act in a manner similar to that of exogenous xylanases, by disrupting cell walls and enhancing contact between digestive enzymes and cell contents in wheat. Secondly, the influence of microbial phytase on the availability of nutrients other than P is now well recognised (Ravindran et al., 2000). It is likely that the observed performance responses in part reflect the release of available amino acids and energy by the added phytase.
Toe ash content, on the other hand, is a good indicator of P availability following the use of phytase since increases in toe ash content can only be caused by improvements in P available for bone mineralization. The toe ash data provide direct evidence for the action of Allzyme Phytase on the release of phytate-bound P (Tables 7 and 8) and demonstrate the effectiveness of Allzyme Phytase in improving availability of P in wheat-soybean meal-based diets for broiler chickens. Phytase supplementation of the low-P diet increased toe ash content to a level comparable to that observed in the adequate-P diets. In male broilers, addition of 500, 1000, 1500 and 2000 U phytase/kg to the low-P diet increased toe ash content by 10.5, 12.3, 11.8 and 13.2%, respectively. The corresponding increases in females were 11.0, 12.6, 14.0 and 14.3%, respectively.
Conclusions
It is concluded, based on performance and toe ash data, that Allzyme Phytase is effective in enhancing utilization of phytate P in wheat-soy diets for broiler chickens. The better performance of birds fed phytase-supplemented diets compared to those fed diets supplemented with inorganic P may, in part, be attributed to side-enzyme activities present in Allzyme Phytase. The present data, along with previous reports (Ravindran et al., 1999: Zyla et al., 1999), suggest that preparations with multiple enzyme activities may provide a competitive strategy to improve nutrient utilization in wheat-based diets for poultry.
References
Authors: V. RAVINDRAN, Y.B. WU, D.V. THOMAS, B.J. CAMDEN, P.C.H. MOREL and W.H. HENDRIKSFrolich, W. 1990. Chelating properties of dietary fiber and phytate. The role for mineral availability. In: New Developments in Dietary Fiber (I. Furda and C.J. Brine, eds), pp. 83-93, Plenum Press, NY.
Kornegay, E.T. 1999. Feeding to reduce nutrient excretion: effects of phytase on phosphorus and other nutrients. In: Biotechnology in the Feed Industry, Proceedings of the15th Annual Symposium (T.P. Lyons and K.A. Jacques, eds), pp. 461-490, Nottingham University Press, Nottingham, UK.
Kornegay, E.T., D.M. Denbow, Z. Yi and V. Ravindran. 1996. Response of broilers to graded levels of Natuphos phytase added to corn-soybean mealbased diets containing three levels of nonphytate phosphorus. Br. J. Nutr. 75:839-852.
Nelson, T.S. 1967. The utilization of phytate P by poultry - a review. Poult. Sci. 46:862–866.
NRC. 1994. Nutrient Requirements of Domestic Animals. Nutrient Requirements of Poultry, 9th rev. ed., National Research Council, National Academy Press, Washington, DC.
Potter, L.M. 1988. Bioavailability of phosphorus from various phosphates based on body weight and toe ash measurements. Poult. Sci. 67:96-102.
Ravindran, V., W.L. Bryden and E.T. Kornegay. 1995. Phytates: occurrence, bioavailability and implications in poultry nutrition. Poult. & Avian Biol. Rev. 6:125-143.
Ravindran, V., P.H. Selle and W.L. Bryden, 1999. Effects of phytase supplementation, individually and in combination, with glycanase, on the nutritive value of wheat and barley. Poult. Sci. 78:1588-1595.
Ravindran, V., P.H. Selle and W.L. Bryden. 2000. Role of microbial phytase in poultry and pig nutrition. Proceedings of the Third European Feed Enzyme Symposium. Amsterdam, the Netherlands (in press).
SAS Institute. 1990. SAS/STAT® User’s Guide: Statistics. Release 6.04 Edition. SAS Institute Inc., Cary, NC.
Yi, Z., E.T. Kornegay, V. Ravindran and D.M. Denbow. 1996. Improving phytate phosphorus availability in corn and soybean meal for broilers using microbial phytase and calculation of phosphorus equivalency values for phytase. Poult. Sci. 75:240-249.
Zyla, K., D. Gogol, J. Koreleski, S. Swiatkiewicz and D.R. Ledoux. 1999. Simultaneous application of phytase and xylanase to broiler feeds based on wheat feeding experiments with growing broilers. J. Sci. Food Agric. 79:1841-1848.
Institute of Food, Nutrition and Human Health, Massey University, Palmerston North, New Zealand








.jpg&w=3840&q=75)