Diagnosis of Gallibacterium Anatis in Layers: First Report in Turkey
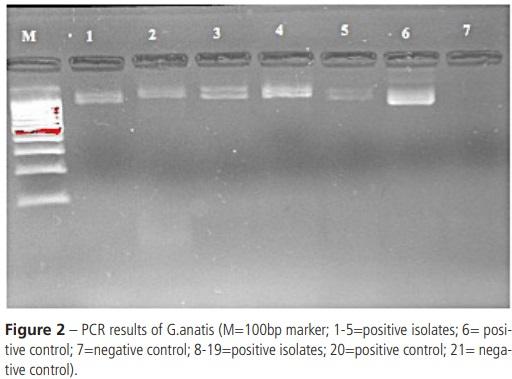
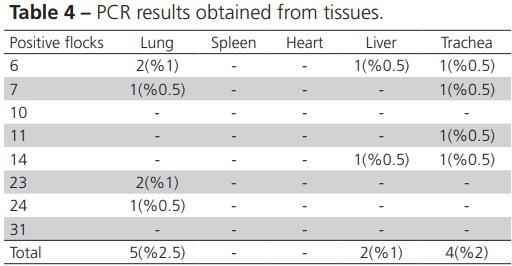
Alispahic M, Christensen H, Hess C, Razzazi-Fazeli E, Bisgaard M, Hess
M. Identification of Gallibacterium species by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry evaluated by multilocus sequence analysis. International Journal of Medical
Microbiology 2011;301(6):513-522.
Ataei S, Bojesen AM, Amininajafi F, Ranjbar MM, Banani M, Afkhamnia M, et al., First report of Gallibacterium isolation from layer chickens in Iran.
Archives of Razi Institute Journal 2017;72(2):123-128.
Aubin GG, Haloun A, Treilhaud M, Reynaud A, Corvec S. Gallibacterium anatis bacteremia in a human. Journal of Clinical Microbiology
2013;51(11):3897-3899.
Bager RJ, Nesta B, Pors S.E, Soriani M, Serino L, Boyce JD, et al., The fimbrial protein flfA from Gallibacteriumanatis is a virulence factor and vaccine candidate. Infection and Immunity 2013;81(6):1964-1973.
Bisgaard M. Incidence of Pasteurella haemolytica in the respiratory tract of apparently healthy chickens and chickens with infectious bronchitis characterisation of 213 strains. Avian Pathology 1977;6(4):285-292.
Bisgaard M, Korczak BM, Busse HJ, Kuhnert P, Bojesen AM. Classification of the taxon 2 and taxon 3 complex of Bisgaard within Gallibacterium and description of Gallibacterium melopsittaci sp. nov., Gallibacterium trehalosifermentans sp. nov. and Gallibacterium salpingitidis sp. nov.
International Journal of Systematic and Evolutionary Microbiology
2009;59(4):735-744.
Bojesen AM. Gallibacterium infection in chickens [thesis]. Denmark (DK):
Department of Veterinary Microbiology the Royal Veterinary and
Agricultural University; 2003.
Bojesen AM, Christensen JP, Nielsen OL, Olsen JE, Bisgaard M. Detection of Gallibacteriumspp. in chickens by fluorescent 16S rRNA in situ hybridization. Journal of Clinical Microbiology 2003;41(11):5167-
5172.
Bojesen AM, Nielsen OL, Christensen JP, Bisgaard M. In vivo studies of Gallibacteriumanatis infection in chickens. Avian Pathology
2004;33(2):145-152.
Bojesen AM, Nielsen SS, Bisgaard M. Prevalence and transmission of haemolytic Gallibacterium species in chicken production systems with different biosecurity levels. Avian Pathology 2003;32(5):503-510.
Bojesen AM, Shivaprasad HL. Genetic diversity of Gallibacterium isolates from California turkey. Avian Pathology 2006;36(3):227-230.
Bojesen AM, Torpdahl M, Christensen H, Olsen JE, Bisgaard M. Genetic diversity of Gallibacterium anatis isolates from different chicken flocks.
Journal of Clinical Microbiology 2003;41(6):2737-2740.
Bojesen AM, Vazquez ME, Bager RJ, Ifrah D, Gonzalez C. Antimicrobial susceptibility and tetracycline resistance determinant genotyping of
Gallibacterium anatis. Veterinary Microbiology 2011;148(1):105-110.
Bojesen AM, Vazquez ME, Robles F, Gonzalez C, Soriano EV, Olsen JE, et al., Specific identification of Gallibacterium by a PCR using primers targeting the 16S rRNA and 23S rRNA genes. Veterinary Microbiology
2007;123(1-3):262-268.
Carlson HC, Whenham GR. Coliform bacteria in chicken broiler house dust and their possible relationship to coli-septicemia. Avian Disease
1968;12(2):297-302.
Chaveza RFO, Barriosa RMM, Chaveza JFH, Mascarenoa JR, Escalantea
JGAI, Yanesb MA. First report of biovar 6 in birds immunized against
Gallibacterium anatis in poultry farms located in Sonora, Mexico.
Veterinaria México Journal 2017;4(3):1-8.
Christensen H, Bisgaard M, Bojesen AM, Mutters R, Olsen JE. Genetic relationships among avian isolates classified as Pasteurella haemolytica,
‘Actinobacillus salpingitidis’ or Pasteurella anatis with proposal of
Gallibacterium anatis gen. nov., comb. nov. and description of 31. additional genomospecies within Gallibacterium gen. nov. International
Journal of Systematic and Evolutionary Microbiology 2003;53(Pt
1):275-287.
Clauer PJ. Why have my hens stopped laying? [cited 2017Apr 29]. Virginia:
Poultry Extension Specialist, Animal and Poultry Sciences; 2009.
Available from: http://www.pubs.ext.vt.edu/content.
El-Adawy H, Bocklisch H, Neubauer H, Hafez HM, Hotzel H. Identification, differentiation and antibiotic susceptibility of Gallibacterium isolates from diseased poultry. Irish Veterinary Journal 2018;71(5):1-10.
Elbestawy AR, Ellakany HF, S Abd El-Hamid H, Bekheet AA, Mataried NE,
Nasr SM, et al., Isolation, characterization, and antibiotic sensitivity assessment of Gallibacterium anatisbiovar heamolytica, from diseased
Egytian chicken flocks during the years 2013 and 2015. Poultry Science
2018;97(5):1519-1525.
Gross WB. The development of ‘‘air sac disease’’. Avian Disease 1961;5:431-
436.
Huangfu H, Zhao J, Yang X, Chen L, Chang H, Wang X, et al., Development and preliminary application of a quantitative PCR assay for detecting gtxA-containing Gallibacterium species in chickens. Avian Disease
2012;56(2):315-320.
Johnson TJ, Danzeisen JL, Trampel D, Nolan LK, Seemann T, Bager RJ, et al.,
Genome analysis and phylogenetic relatedness of Gallibacterium anatis strains from poultry. PloS One 2013;8(1):1-12.
Jones KH, Thornton JK, Zhang Y, Mauel MJ. A 5-year retrospective report of
Gallibacterium anatis and Pasteurella multocida isolates from chickens in Mississippi. Poultry Science 2013;92(12):3166-3171.
Lawal JR, Ndahi JJ, Dauda J, Gazali YA, Gadzama JJ, Aliyu AU. Survey of
Gallibacterium anatis and its antimicrobial susceptibility pattern in village chickens (Gallus gallus domesticus) in Maiduguri, North-eastern
Nigeria. International Journal of Veterinary Science and Medicine
2018;1(1):1-7.
Mirle C, Schöngarth M, Meinhart H, Olm U. Studies into incidence of Pasteurella haemolytica infections and their relevance to hens, with particular re- ference to diseases of the egg-laying apparatus.
Monatsheftefuer Veterinaermedizin 1991;45:545-549.
Mushin R, Weisman Y, Singer N. Pasteurella haemolytica found in the respiratory tract of fowl. Avian Disease 1979;24(1):162-168.
Neubauer C, Souza-Pilz MD, Bojesen AM, Bisgaard M, Hess M. Tissue distribution of haemolytic Gallibacterium anatis isolates in laying birds with reproductive disorders. Avian Pathology 2009;38(1):1-7.
Paudel S, Alispahic M, Liebhart D, Hess M, Hess C. Assessing pathogenicity of Gallibacterium anatis in a natural infection model: the respiratory and reproductive tracts of chickens are targets for bacterial colonization.
Avian Pathology 2013;42(6):527-535.
Paudel S, Liebhart D, Aurich C, Hess M, Hess C. Pathogenesis of
Gallibacterium anatis in a natural infection model fulfils Koch’s postulates: 2. Epididymitis and decreased semen quality are the predominant effects in specific pathogen free cockerels. Avian
Pathology 2014;43(6):529-534.
Paudel S, Liebhart D, Hess M, Hess C. Pathogenesis of Gallibacterium anatis in a natural infection model fulfils Koch’s postulates: 1. Folliculitis and drop in egg production are the predominant effects in specific pathogen free layers. Avian Pathology 2014;43(5):443-449.
Persson G, Bojesen AM. Bacterial determinants of importance in the virulence of Gallibacterium anatis in poultry. Veterinary Research
2015;46(1):1-11.
Rzewuska M, Karpinska E, Szeleszczuk P, Binek M. Isolation of
Gallibacterium spp. from peacocks with respiratory tract infections.
Medycyna Weterynaryjna 2007;63(11):1431-1433.
Sambrook J, Green MR. Molecular cloning: a laboratory manual. 4th ed.
Cold Spring Harbor: Cold Spring Harbor Laboratory; 2012.
Sing SV. Studies on growth kinetics of Gallibacterium anatis in presence of deuterium oxide (D2O, heavy water) [thesis]. Izatnagar: Deemed
University ICAR-Indian Veterinary Research Institute; 2016.
Singh SV, Singh BR, Sinha DK, Vinodh K, Prasanna VA, BhardwajM, et al., Gallibacterium anatis: an emerging pathogen of poultry birds and domiciled birds. Journal of Veterinary Science and Technology
2016;7(3):1-7.
Sorour HK, Al Atfeehy NM, Shalaby AG. Gallibacterium anatis infection in chickens and ducks. Assiut Veterinary Medical Journal 2015;61(147):80-
86.
Yum-Bir. Poultry sectoral data [cited 2018 Nov 6]. Ankara; 2017. Available from: http://www.yumbir.org/userfiles/file.



United States








