INTRODUCTION
Salmonellae including pullorum disease, fowl typhoid and other infections may cause varieties of clinical signs from acute systemic disease and gastrointestinal symptoms in poultry flocks to embryonic problem in hatchery. With great expansion of the poultry rearing and farming, pullorum disease and fowl typhoid have become wide spread problem in Bangladesh as well as other countries of the world. In recent years, diagnostic laboratories have been concerned with reducing the time required for diagnosis of Salmonella infections. The current standard laboratory procedure to culture and identify Salmonella takes approximately 4 to 7 days. Even these methods are tedious, time consuming and confer little guarantee of sensitivity and species specificity. Only early diagnosis of the disease could be the cornerstone of the control program. For this, tube agglutination test for pullorum disease was described by Jones in 1913 and subsequent introduction of a rapid plate serum agglutination (RSA) test and a stained antigen for whole blood test provided a practical basis. But none of these tests were found sensitive and was solved by the development of the enzyme-linked-immuno-sorbent assay (ELISA) and its application to the measurement of antibody response to specific infections. The application of ELISA assays for Salmonella enteritidis have been described. However, ELISA test is expensive, time consuming, needs skilled manpower and not easy to perform in the field condition.
The micro agglutination system had been adapted for a wide variety of serological procedures due to its better sensitivity. It has been cited for detecting agglutinations of several Salmonella serotypes in the field condition as well as a routine diagnostic test for the detection of chronic carriers of S. pullorum and S. gallinarum. Antigen produce from local isolate is always more sensitive and will be economically cheaper than the imported one. Therefore, the present study was undertaken with the following objective: Preparation of stained colored Salmonella antigen with a local isolate and development of slide micro agglutination system for the rapid diagnosis of Salmonella infection in chickens in the field condition.
MATERIALS AND METHODS
Isolation and Identification of Salmonella
The locally isolated S. pullorum was used for production of the Salmonella colored antigen. Test tubes containing samples on nutrient broth were incubated for 24 hrs at 37°C. From the nutrient broth, subcultures were also made on Brilliant Green agar, Salmonella Shigella agar, MacConkey agar, EMB agar, TSI agar, LB agar and nutrient agar, and incubated at 37°C for over night. The identification of the organisms was performed by the tests as described by different literature. On the basis of colony and staining characters the organisms were isolated and identified. The organism was further confirmed by PCR as described in.
Preparation of Salmonella stained color antigen
A conical flask was taken, containing 50 ml of LB broth. Single colony of Salmonella pullorum was inoculated into the culture. Then the flasks were placed in incubation for 48 hrs. 50 ml broth culture was divided into two conical flasks containing each 25ml.Then tetrazolium salt was added aseptically in the amount of 0.5% in each broth culture and incubated one flask for 2 hrs and another flask for 24 hrs. After incubation, 0.5% phenol was added and incubated for 1 hr and 2 hrs, espectively. The stained broth suspension was filtered through sterile gauge and poured in to eppendorf tube. Afterwards, these were centrifuged at 16000 rpm for 15 minutes, supernatant was decanted and cells were suspended in 0.5% phenolized saline, 0.5% fomalinized saline and 0.09% sodium azide. The suspension was mixed by vigorous vortex with a few sterile small pieces of glass and transferred it another eppendorf tube. Then the solution was stored at 4°C as neotetrazolium stained antigen for future use. The total protein concentration of the stained antigen was measured by the Folin Phenol method of Lowry.
Development of slide micro-agglutination test
For this, 20 μl of stained antigen and 20 μl chicken sera were placed on a sterile glass slide by a micropipette and mixed thoroughly by stirring with tips. The results were read within 1 minute. In positive case, granules (agglutinates) were formed rapidly due to combination of homologous antigen and antibody which was seen during rocking. In the absence of antibody, no such granules (agglutinates) were formed. Positive and negative sera were run parallel in each time.
Calculation of the titer of the antibody
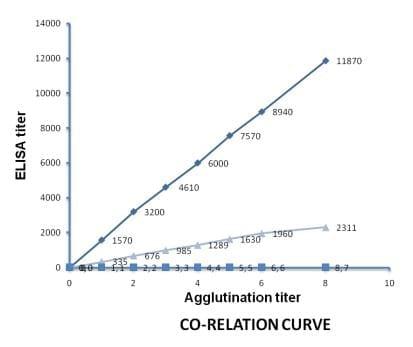
The known positive sera were obtained from the Department of Pathology, Bangladesh Agricultural University, Mymensingh that was raised. The sera were then diluted and the results of diluted sera were observed. The known ELISA titers of positive sera ranging from 676 to 1, 2000 were selected for the 10 fold or 2 fold dilutions. The agglutination titer of each diluted sera was compared with the known ELISA titer and plotted in standard curves. Co-relation curve was prepared using 10 fold and 2 fold diluted sera to determine the antibody titer.
Validation with field sample
A total of 180 sera from 7 flocks and 4 birds (3 dead and 1 live) from one flock were donated by a commercial hatchery of Bangladesh. Necropsy and histopathology was done on the selected bird sample and the findings were recorded. The slide micro-agglutination test was performed with the newly developed salmonella colored antigen. ELISA titers of field sera were determined by the help of standard curve.
Storage and shelf life determination
To determine the shelf life of stained antigen three preservatives 0.5% phenolized saline, 0.5% formalinized saline and 0.09% sodium azide were chosen and observed for several months during the study period. The stained antigen was then stored at 4°C and also observed the storage temperature.
Packaging
For easy dispensing of salmonella colored antigen to the field users a package was prepared. The prepared package contains 1ml stained antigen in a glass vial, positive serum, negative serum and information sheet. Materials not supplied but are necessary are glass slide for slide agglutination test, dropper, and stirrer.
RESULTS AND DISCUSSION
Preparation of Salmonella colored antigen
In the present study a sensitive neotetrazolium stained Salmonella pullorum antigen was prepared from local isolates and a micro-agglutination procedure for the detection of S. pullorum antibody was developed. The protein concentration of the colored antigen was adjusted to 3000ng/µl with the help of BSA standard curve. The tetrazolium stained Salmonella antigen showed enhanced sensitivity than the conventional agglutination test as previously described. Highest sensitivity was recorded with the antigen developed from 48 hr LB broth culture. The increased sensitivity of the antigen probably due to it has been produced from local isolates and also may be due to modification of the preparation procedure.
Development of micro-agglutination test
The slide micro-agglutination test was performed as per procedure. The results were read within 1 minute. In positive case, granules (agglutinates) were formed rapidly due to combination of homologous antigen and antibody which was seen during rocking. In the absence of antibody, no such granules (agglutinates) were formed. The reaction started to develop within 30 seconds and completed within 1 minute. The color of the agglutinins was pink and agglutinins were fine granular, (Figure 1 and 2). Since the agglutinins were suspended on the colorless fluid (serum), these were easily visible by the naked eye without the problems of being doubtful and cloudy reactions which is so common with conventional agglutination test. The specificity of the stained antigen was high as it did not react with the negative control serum and water.
Figure 1: Positive agglutination with three known positive sera
Figure 2: Agglutination results with both positive and negative sera
Preservative
Suitable preservatives were chosen and agglutination test was performed with some known positive sera and the results were observed in every 2 months interval. It was observed that the 0.5% phenolized saline give better result than other chosen preservatives.
Determination of antibody titer
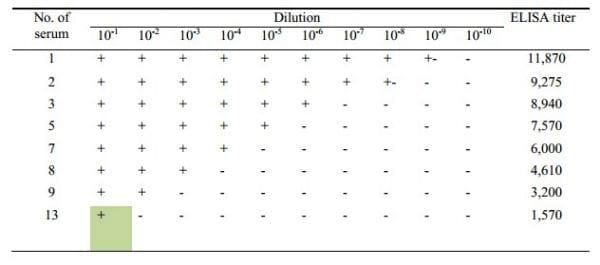
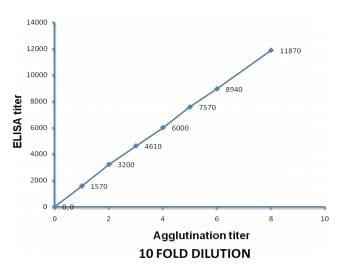
The agglutination results of the 10 fold diluted sera are shown in the Table 1. In 10 fold dilution, the positive agglutination was directly proportional to the titer of the antibodies (Figure 3). The positive agglutination was recorded maximum up to 10-8 dilution with antibody having titer 11870, while it was up to 10-1 dilution when antibody titer was 1570. To check it further, 2 fold dilutions were made from a panel of antibodies having ELISA titer 335-2311.
Table 1: Agglutination results with 10-fold diluted sera showing agglutination with diluted positive sera of different titer
Figure 3: Proportional curve between 10 fold agglutination and ELISA titer
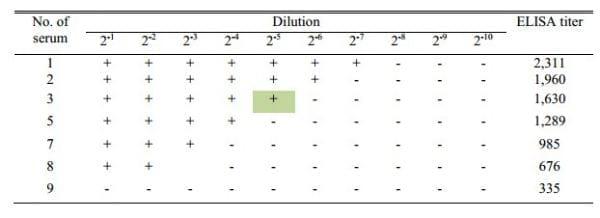
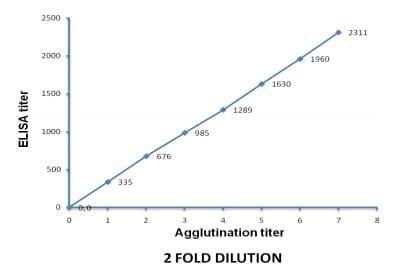
The agglutination results of the 2 fold diluted sera are shown in Table 2. The agglutination was again directly proportional to the titer of the antibodies (Figure 4). The positive agglutination recorded to maximum of up to 2-7 dilution with antibody having titer 2311, while it was up to 2-2 dilution with antibody having titer 676 and no agglutination with antibodies having titer 335 (Table 2). The results showed that serum number 13 of Table 1 and serum number 3 of Table 2 had almost similar ELISA titer and their agglutination titer (10-1 or 2-5) also similar.
Table 2: Agglutination results with 2 fold diluted sera showing agglutination with diluted positive sera of different titer
Figure 4: Proportional curve between 2 fold agglutination and ELISA titer
Standard curve
From the 10 fold and 2 fold dilution, it was observed that the end point agglutination titer of serum sample having ELISA titer of 1,570 was found in 10-1th dilution, while in 2 fold dilution end point agglutination titer was found in 2-5th dilution with serum sample having ELISA titer 1,630. This indicates a positive co-relation between 2 and 10 fold dilution (Figure 5).
Figure 5: Standard curve between ELISA and agglutination titer (10 fold and 2 fold). Series 1: 10 fold agglutination titer and ELISA titer, Series 2: Dilution scale, Series 3: 2 fold agglutination titer and ELISA titer
Validation with field sample
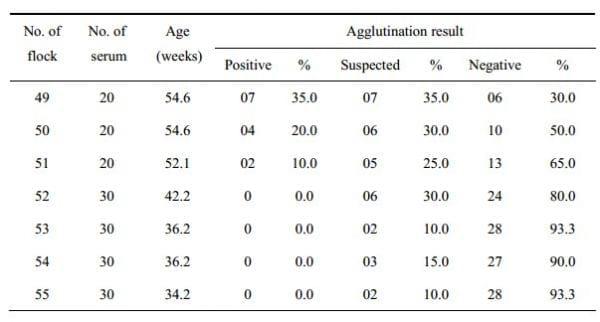
Among the 7 flocks, the flock number 49 showed the higher prevalence as 35% confirmed case, 35% suspected and 30% negative. The flock number 50 and 51 also showed positive agglutination in 20% and 10% cases, respectively. The flock number 52, 53, 54 and 55 did not show any positive result but 30, 10, 15, and 10% cases respectively, were found suspected. Detailed of the results may be seen in Table 3. The agglutination percentage of total number of sera from 7 flocks was also calculated. Among 180 sera it was showed that 13 sera were confirmed as positive, 31 suspected and 76 negative.
Table 3: Field sample showing the agglutination result with newly developed stained antigen
Determination of ELISA titer
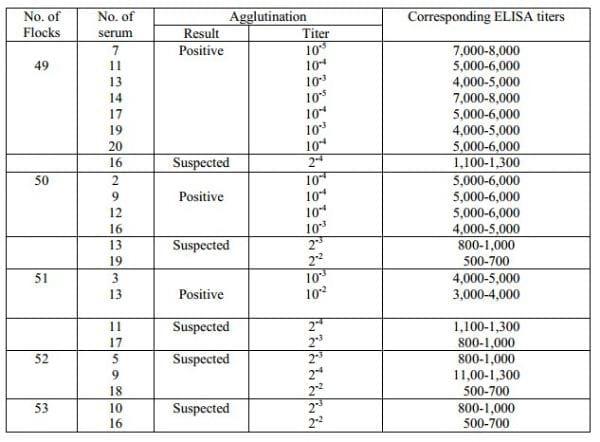
Among the sera tested, 7 positive sera from flock number 49, 4 from 50, 2 from 51 were diluted. Slide micro-agglutination test was performed with the diluted sera. The ELISA titer was calculated from the standard curve.The positive sera from flock number 49 showed agglutination titer at the 10-5th, 10-4th and 10-3th dilution and the corresponding ELISA titer was between 7,000-8,000, 5,000-6,000 and 4,000-5,000, respectively. So it was found that the highest ELISA titer was determined in the flock number 49, that ranged from 4000-8000. Again the positive sera from the flock number 50 and 51 showed agglutination titer at the 10-4th, 10-3th and 10-2th dilution where corresponding ELISA titer was ranged between 5,000-6,000, 4,000-5,000 and 3,000-4,000, respectively. Detail may be seen in Table 4. Otherwise the suspected sera among the flock number 49-53 showed positive agglutination titer at the 2-4th, 2-3th and 2-2th dilution indicating that the ELISA titer was between 1,100-1,300, 800-1,000 and 500-700, respectively. So it was observed that the ELISA titer of suspected sera ranged from 500-1300. The instruction of the ELISA kit (GUILDHAY, UK) also designated the sera having ELISA titer betwen 500-1,500 as suspected.
Table 4: Calculated ELISA titer of the field sera
Sensitivity
A panel of anti-sera was used to check the sensitivity of developed test. The titer of the sera was determined in another study using a commercial ELISA kit (GUILDHAY, UK). As per instruction of the kit, the sample that has ELISA titer >1,500 is considered as positive, titer in between 500-1,500 is considered as suspicious and >500 is considered as negative. In this study, stained antigen produced agglutinins with sera having ELISA titer 676; indicating developed slide agglutination test is more sensitive than ELISA test. However for more confirmation, it can be considered that, when this stained antigen produces agglutinins with only undiluted sera, then it will be designated as suspicious. But if it produces agglutinins with 10 fold diluted sera, then the suspected will be confirmed as salmonella positive.
Specificity
The micro-agglutination technique has distinct advantages in routine serological detection of pullorum disease and fowl typhoid in the poultry. The tetrazolium stained Salmonella antigen from a local isolate was successfully developed which could be used to screen the Salmonella infection in the poultry flocks at the farm premises. Although this antigen was developed from the local isolate of S.10 pullorum it also reacts with antibodies of Salmonella gallinarum and other S. Entriditis. This method also may be used to calculate the titre of the anti-Salmonella antibody in the infected and vaccinated chickens under farm conditions.
CONCLUSION
The tetrazolium stained Salmonella antigen from a local isolate was successfully developed which will help to detect salmonella infection in the chicken flocks in field condition within a very short time. A kit was organized named “BAU-Path S Antigen Kit” contain 1ml of developed stained antigen that was sufficient for 50 test, positive serum, negative serum and information sheet. However; cautions have to be taken with the reading of the results. With increase time, the stained antigen may react with the non-specific antibody present in the serum. Therefore, results within one minute were suggested as confirmed case of salmonellosis. The antigen should be shaking before use. Always keep away from the light.
REFERENCES
Gast RK 1997. Paratyphoid infections. pp 97-121. In: Calnek BW, Barnes HJ, Beard CW, McDoughand LR, Saif YM (eds). Diseases of Poultry, 10th ed. Lowa State University Press. Ames, IA.
Sarker AJ 1976. The prevalence of avian diseases in Bangladesh Agricultural University poultry farm. Bangladesh Vet J 10: 61-66.
Rahman MM, Chowdhury TIMF, Rahman MM, Hossain WIMA 1979. Surveillance of Salmonella and Escherichia organisms in poultry feed. Bangladesh Vet J 15: 59-62.
Rettger LF 1900. Septicaemia among young chickens. NY Med J 71: 803-805.
Runnels RA, Coon CJ, Farley H, Thorp F 1927. An application of the rapid-method agglutination test to the diagnosis of bacillary white diarrhoea infection. J Am Vet Med Assoc 70: 660-662.
Schaffer JM, McDonald AD, Hall WJ, Bunyea H 1931. A stained antigen for the rapid whole blood test for pullorum disease. J Am Vet Med Assoc 79: 236-240
Engvall E, Perlman P 1971. Enzyme-linked immunosorbent assay(ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 8(9): 871-874.
Voller A, Bidwell D, Bartlett A 1976. Microplate enzyme immunoassays for the immuno-diagnosis of virus infections. pp 506-512. Manual of Clinical Immunology, ASM, Washington DC.
Cooper GL, Nicholas RA, Bracewel CD 1989. Serological and bacteriological investigations of chickens from flocks naturally infected with Salmonella enteritidis. Vet Rec 125(23): 567-572.
Timoney JF, Sikora N, Shivaprasad HL, Opitz M 1990. Detection of antibody to Salmonella enteritidis by a gm flagellin-based ELISA. Vet Rec 127(7): 168-169.
Nicholas RA, Cullen GA 1991. Development and application of an ELISA for detecting antibodies to Salmonella enteritidis in chicken flocks. Vet Rec 128: 74-76.
Anonymous 1970. A selected bibliography of micromethods in microbiology with special emphasis on microtiter techniques. Cooke Engineering Co, Alexandria, Va.
Witlin B 1967. Detection of antibodies by microtitration techniques. Mycopathol Mycol Appl 33(3): 241-257.
Williams JE, Whitemore AD 1970. Serological diagnosis of pullorum disease with the microagglutination system. Appl Microbiol 21(3): 394-399.
Merchant IA, Packer RA 1967. Veterinary Bacteriology and Virology. pp 211-305. 7thed. Iowa State University Press, Ames, Iowa, USA.
Freeman BA 1985. Burrow’s Text Book of Microbiology. pp 372-472. 22nd ed. WB Saunders Company, London, UK
Cheesbrough M 2000. District Laboratory Practice in Tropical Countries, part 2. pp 64-65.Cambridge low price ed. Cambridge University Press, UK.
Brooks GF, Butel JS, Morse SA 2002. Jawetz, Melnick and Adelberg’s Medical Microbiology. pp 197-202. 22nd (international) ed. McGraw Hill, New Delhi, India.
Saha SS, Chowdhury EH, Rhaman SM, Sultana S, Haider MG, Islam MR 2007 Detection of Salmonella gallinarum using polymerase chain reaction. Paper submitted to Bangladesh Veterinary Journal.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ 1951 Protein measurement with the polin phenol reagent. J Biol Chem 193(1): 265-275
Williams JE, Whittemore AD 1972 Microantiglobulin test for detecting Salmonella typhimurium agglutinins. Appl Microbiol 23(5): 931-937.