Determination of Chlortetracycline Residues, Antimicrobial Activity and Presence of Resistance Genes in Droppings of Experimentally Treated Broiler Chickens
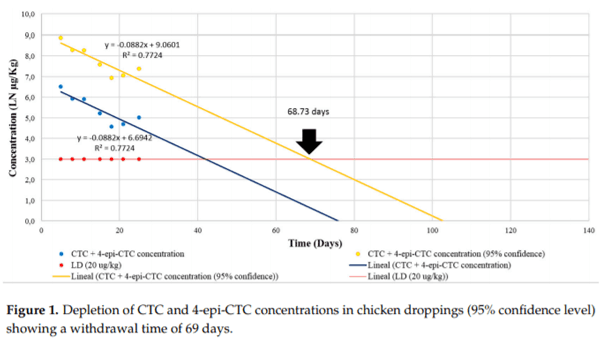
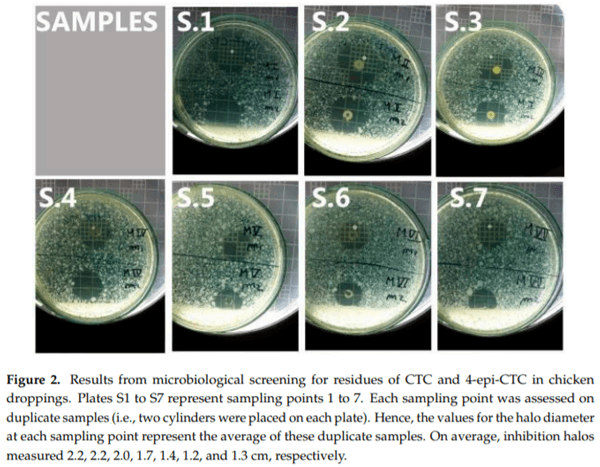
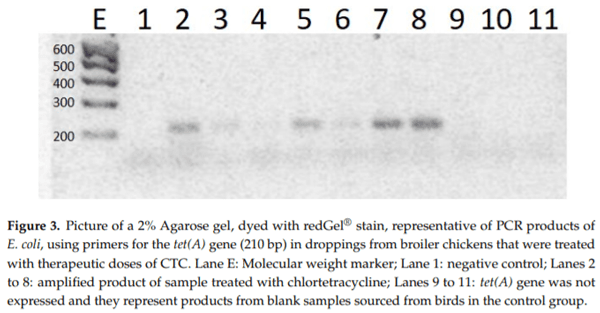
Tetracyclines are important antimicrobial drugs for poultry farming that are actively excreted via feces and urine. Droppings are one of the main components in broiler bedding, which is commonly used as an organic fertilizer. Therefore, bedding becomes an unintended carrier of antimicrobial residues into the environment and may pose a highly significant threat to public health. For this depletion study, 60 broiler chickens were treated with 20% chlortetracycline (CTC) under therapeutic conditions. Concentrations of CTC and 4-epi-CTC were then determined in their droppings. Additionally, this work also aimed to detect the antimicrobial activity of these droppings and the phenotypic susceptibility to tetracycline in E. coli isolates, as well as the presence of tet(A), tet(B), and tet(G) resistance genes. CTC and 4-epi-CTC concentrations that were found ranged from 179.5 to 665.8 g/kg. Based on these data, the depletion time for chicken droppings was calculated and set at 69 days. All samples presented antimicrobial activity, and a resistance to tetracyclines was found in bacterial strains that were isolated from these samples. Resistance genes tet(A) and tet(B) were also found in these samples.
Keywords: tetracycline residues; depletion; poultry droppings; antimicrobial activity; antimicrobial resistance genes.
- Tasho, R.P.; Cho, J.Y. Veterinary antibiotics in animal waste, its distribution in soil and uptake by plants: A review. Sci. Total Environ. 2016, 563–564, 366–376. [CrossRef] [PubMed]
- European Parliament and the Council of the European Union No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union 2003, 268, 29–43.
- Jeong, J.; Song, W.; Cooper, W.J.; Jung, J.; Greaves, J. Degradation of tetracycline antibiotics: Mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere 2010, 78, 533–540. [CrossRef] [PubMed]
- Kim, K.-R.; Owens, G.; Kwon, S.-I.; So, K.-H.; Lee, D.-B.; Ok, Y.S. Occurrence and environmental fate of veterinary antibiotics in the terrestrial environment. Water Air Soil Pollut. 2011, 214, 163–174. [CrossRef]
- Del Castillo, J.R.E. Tetracyclines. In Antimicrobial Therapy in Veterinary Medicine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 257–268. ISBN 978-1-118-67501-4.
- Fairchild, A.S.; Smith, J.L.; Idris, U.; Lu, J.; Sanchez, S.; Purvis, L.B.; Hofacre, C.; Lee, M.D. Effects of Orally Administered Tetracycline on the Intestinal Community Structure of Chickens and on tet Determinant Carriage by Commensal Bacteria and Campylobacter jejuni. Appl. Environ. Microbiol. 2005, 71, 5865–5872. [CrossRef] [PubMed]
- Jacob, J. Antibiotics Approved for Use in Conventional Poultry Production. Available online: http://articles.extension.org/pages/66981/antibiotics-approved-for-use-in-conventional-poultry-production (accessed on 9 March 2018).
- Landoni, M.; Albarellos, G. The use of antimicrobial agents in broiler chickens. Vet. J. 2015, 205, 21–27. [CrossRef] [PubMed]
- Massé, D.I.; Saady, N.M.C.; Gilbert, Y. Potential of biological processes to eliminate antibiotics in livestock manure: An overview. Animals 2014, 4, 146–163. [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [CrossRef]
- Martínez-Carballo, E.; González-Barreiro, C.; Scharf, S.; Gans, O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environ. Pollut. 2007, 148, 570–579. [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Li, W.; Lu, X.; Liu, B.; Wang, J. The residues and environmental risks of multiple veterinary antibiotics in animal faeces. Environ. Monit. Assess. 2013, 185, 2211–2220. [CrossRef] [PubMed]
- Hou, J.; Wan, W.; Mao, D.; Wang, C.; Mu, Q.; Qin, S.; Luo, Y. Occurrence and distribution of sulfonamides, tetracyclines, quinolones, macrolides, and nitrofurans in livestock manure and amended soils of Northern China. Environ. Sci. Pollut. Res. 2015, 22, 4545–4554. [CrossRef] [PubMed]
- Zhang, H.; Luo, Y.; Wu, L.; Huang, Y.; Christie, P. Residues and potential ecological risks of veterinary antibiotics in manures and composts associated with protected vegetable farming. Environ. Sci. Pollut. Res. 2015, 22, 5908–5918. [CrossRef] [PubMed]
- Carballo, M.; Aguayo, S.; González, M.; Esperon, F.; de la Torre, A. Environmental Assessment of Tetracycline’s Residues Detected in Pig Slurry and Poultry Manure. J. Environ. Prot. 2016, 7, 82–92. [CrossRef]
- Van Epps, A.; Blaney, L. Antibiotic Residues in Animal Waste: Occurrence and Degradation in Conventional Agricultural Waste Management Practices. Curr. Pollut. Rep. 2016, 2, 135–155. [CrossRef]
- Eisner, H.; Wulf, R. The metabolic fate of chlortetracycline and some comparisons with other tetracyclines. J. Pharmacol. Exp. Ther. 1963, 142, 122–131. [PubMed]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [CrossRef] [PubMed
- Chan, K.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Aust. J. Soil Res. 2008, 46, 437–444. [CrossRef]
- Furtula, V.; Farrell, E.G.; Diarrassouba, F.; Rempel, H.; Pritchard, J.; Diarra, M.S. Veterinary pharmaceuticals and antibiotic resistance of Escherichia coli isolates in poultry litter from commercial farms and controlled feeding trials. Poult. Sci. 2010, 89, 180–188. [CrossRef] [PubMed]
- Leal, R.M.P.; Figueira, R.F.; Tornisielo, V.L.; Regitano, J.B. Occurrence and sorption of fluoroquinolones in poultry litters and soils from São Paulo State, Brazil. Sci. Total Environ. 2012, 432, 344–349. [CrossRef] [PubMed]
- SAG. Diagnosis of the Environmental Problems Caused by Poultry and Dairy Livestock in Chile, and Training on Assessment of Animal Farms; Servicio Agrícola y Ganadero: Santiago, Chile, 2006.
- Sanchuki, C.E.; Soccol, C.R.; de Carvalho, J.C.; Soccol, V.T.; do Nascimento, C.; Woiciechowski, A.L. Evaluation of poultry litter traditional composting process. Braz. Arch. Biol. Technol. 2011, 54, 1053–1058. [CrossRef]
- Yang, Q.; Zhang, H.; Guo, Y.; Tian, T. Influence of Chicken Manure Fertilization on Antibiotic-Resistant Bacteria in Soil and the Endophytic Bacteria of Pakchoi. Int. J. Environ. Res. Public Health 2016, 13, 662. [CrossRef] [PubMed]
- Pan, M.; Chu, L. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total Environ. 2017, 599, 500–512. [CrossRef] [PubMed]
- Kwon, S.; Owens, G.; Ok, Y.; Lee, D.; Jeon, W.-T.; Kim, J.; Kim, K.-R. Applicability of the Charm II system for monitoring antibiotic residues in manure-based composts. Waste Manag. 2011, 31, 39–44. [CrossRef] [PubMed]
- Nõlvak, H.; Truu, M.; Kanger, K.; Tampere, M.; Espenberg, M.; Loit, E.; Raave, H.; Truu, J. Inorganic and organic fertilizers impact the abundance and proportion of antibiotic resistance and integron-integrase genes in agricultural grassland soil. Sci. Total Environ. 2016, 562, 678–689. [CrossRef] [PubMed]
- Chen, Z.; Jiang, X. Microbiological safety of chicken litter or chicken litter-based organic fertilizers: A review. Agriculture 2014, 4, 1–29. [CrossRef]
- Hu, X.; Zhou, Q.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ. Pollut. 2010, 158, 2992–2998. [CrossRef] [PubMed]
- Bassil, R.J.; Bashour, I.I.; Sleiman, F.T.; Abou-Jawdeh, Y.A. Antibiotic uptake by plants from manure-amended soils. J. Environ. Sci. Health Part B 2013, 48, 570–574. [CrossRef] [PubMed]
- Chung, H.S.; Lee, Y.-J.; Rahman, M.M.; Abd El-Aty, A.M.; Lee, H.S.; Kabir, M.H.; Kim, S.W.; Park, B.-J.; Kim, J.-E.; Hacimüftüoglu,? F.; et al. Uptake of the veterinary antibiotics chlortetracycline, enrofloxacin, and sulphathiazole from soil by radish. Sci. Total Environ. 2017, 605–606, 322–331. [CrossRef] [PubMed]
- Liu, F.; Ying, G.-G.; Tao, R.; Zhao, J.-L.; Yang, J.-F.; Zhao, L.-F. Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities. Environ. Pollut. 2009, 157, 1636–1642. [CrossRef] [PubMed]
- Hillis, D.G.; Fletcher, J.; Solomon, K.R.; Sibley, P.K. Effects of ten antibiotics on seed germination and root elongation in three plant species. Arch. Environ. Contam. Toxicol. 2011, 60, 220–232. [CrossRef] [PubMed]
- Minden, V.; Deloy, A.; Volkert, A.M.; Leonhardt, S.D.; Pufal, G. Antibiotics impact plant traits, even at small concentrations. AoB Plants 2017, 9. [CrossRef] [PubMed]
- Park, S.; Choi, K. Hazard assessment of commonly used agricultural antibiotics on aquatic ecosystems. Ecotoxicology 2008, 17, 526–538. [CrossRef] [PubMed]
- Ji, K.; Kim, S.; Han, S.; Seo, J.; Lee, S.; Park, Y.; Choi, K.; Kho, Y.-L.; Kim, P.-G.; Park, J.; et al. Risk assessment of chlortetracycline, oxytetracycline, sulfamethazine, sulfathiazole, and erythromycin in aquatic environment: Are the current environmental concentrations safe? Ecotoxicology 2012, 21, 2031–2050. [CrossRef] [PubMed]
- Kolodziejska, M.; Maszkowska, J.; Bialk-Bielinska,´ A.; Steudte, S.; Kumirska, J.; Stepnowski, P.; Stolte, S. Aquatic toxicity of four veterinary drugs commonly applied in fish farming and animal husbandry. Chemosphere 2013, 92, 1253–1259. [CrossRef] [PubMed]
- Havelkova, B.; Beklova, M.; Kovacova, V.; Hlavkova, D.; Pikula, J. Ecotoxicity of selected antibiotics for organisms of aquatic and terrestrial ecosystems. Neuroendocrinol. Lett. 2016, 37, 38–44. [PubMed]
- CVMP. Guideline on Approach towards Harmonisation of Withdrawal Periods; European Medicines Agency, Committee for Veterinary Medicinal Products: London, UK, 2016; p. 37.
- Berendsen, B.J.; Wegh, R.S.; Memelink, J.; Zuidema, T.; Stolker, L.A. The analysis of animal faeces as a tool to monitor antibiotic usage. Talanta 2015, 132, 258–268. [CrossRef] [PubMed]
- Sumano López, H.S.; Gutiérrez Olvera, L. Farmacología Clínica en aves Comerciales [Clinical Pharmacology in Poultry], 4th ed.; Interamericana Mc-Graw-Hill: Mexico Df, Mexico, 2010.
- Cornejo, J.; Pokrant, E.; Krogh, M.; Briceño, C.; Hidalgo, H.; Maddaleno, A.; Araya-Jordán, C.; Martín, B.S. Determination of oxytetracycline and 4-epi-oxytetracycline residues in feathers and edible tissues of broiler chickens using liquid chromatography coupled with tandem mass spectrometry. J. Food Prot. 2017, 80, 619–625. [CrossRef] [PubMed]
- Berendsen, B.J.; Bor, G.; Gerritsen, H.W.; Jansen, L.J.; Zuidema, T. The disposition of oxytetracycline to feathers after poultry treatment. Food Addit. Contam. Part A 2013, 30, 2102–2107. [CrossRef] [PubMed]
- Cornejo, J.; Pokrant, E.; Araya, D.; Briceño, C.; Hidalgo, H.; Maddaleno, A.; Araya-Jordán, C.; San Martin, B. Residue depletion of oxytetracycline (OTC) and 4-epi-oxytetracycline (4-epi-OTC) in broiler chicken’s claws by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Food Addit. Contam. Part A 2017, 34, 494–500. [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [CrossRef] [PubMed]
- Przenioslo-Siwczynska,´ M.; Kwiatek, K. Evaluation of multi-plate microbial assay for the screening of antibacterial substances in animal feedingstuffs. Bull. Vet. Inst. Pulawy 2007, 51, 599–602.
- Kirbiš, A. Microbiological screening method for detection of aminoglycosides, -lactames, macrolides, Tetracyclines and quinolones in meat samples. Slov. Vet. Res. 2007, 44, 11–18.
- Pikkemaat, M.G.; Rapallini, M.L.; Oostra-van Dijk, S.; Elferink, J.A. Comparison of three microbial screening methods for antibiotics using routine monitoring samples. Anal. Chim. Acta 2009, 637, 298–304. [CrossRef] [PubMed]
- Montforts, M.H.; Kalf, D.F.; van Vlaardingen, P.L.; Linders, J.B. The exposure assessment for veterinary medicinal products. Sci. Total Environ. 1999, 225, 119–133. [CrossRef]
- Surette, M.D.; Wright, G.D. Lessons from the Environmental Antibiotic Resistome. Annu. Rev. Microbiol. 2017, 71, 309–329. [CrossRef] [PubMed]
- Tien, Y.-C.; Li, B.; Zhang, T.; Scott, A.; Murray, R.; Sabourin, L.; Marti, R.; Topp, E. Impact of dairy manure pre-application treatment on manure composition, soil dynamics of antibiotic resistance genes, and abundance of antibiotic-resistance genes on vegetables at harvest. Sci. Total Environ. 2017, 581–582, 32–39. [CrossRef] [PubMed]
- Wang, N.; Yang, X.; Jiao, S.; Zhang, J.; Ye, B.; Gao, S. Sulfonamide-Resistant Bacteria and Their Resistance Genes in Soils Fertilized with Manures from Jiangsu Province, Southeastern China. PLoS ONE 2014, 9, e112626. [CrossRef] [PubMed]
- Wang, F.-H.; Qiao, M.; Chen, Z.; Su, J.-Q.; Zhu, Y.-G. Antibiotic resistance genes in manure-amended soil and vegetables at harvest. J. Hazard. Mater. 2015, 299, 215–221. [CrossRef] [PubMed]
- Xie, X.; Zhou, Q.; He, Z.; Bao, Y. Physiological and potential genetic toxicity of chlortetracycline as an emerging pollutant in wheat (Triticum aestivum L.). Environ. Toxicol. Chem. 2010, 29, 922–928. [CrossRef] [PubMed]
- Xie, X.; Zhou, Q.; Bao, Q.; He, Z.; Bao, Y. Genotoxicity of tetracycline as an emerging pollutant on root meristem cells of wheat (Triticum aestivum L.). Environ. Toxicol. 2011, 26, 417–423. [CrossRef] [PubMed]
- European Parliament and the Council of the European Union Directive 2010/63/EU of 22 September 2010 of the European Parliament and of the Council on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–79.
- Pikkemaat, M.G.; Rapallini, M.L.; Zuidema, T.; Elferink, J.W.A.; Oostra-van Dijk, S.; Driessen-van Lankveld, W.D.M. Screening methods for the detection of antibiotic residues in slaughter animals: Comparison of the European Union Four-Plate Test, the Nouws Antibiotic Test and the Premi®Test (applied to muscle and kidney). Food Addit. Contam. Part A 2011, 28, 26–34. [CrossRef] [PubMed]
- Gaudin, V.; Hedou, C.; Rault, A.; Verdon, E. Validation of a Five Plate Test, the STAR protocol, for the screening of antibiotic residues in muscle from different animal species according to European Decision 2002/657/EC. Food Addit. Contam. Part A 2010, 27, 935–952. [CrossRef] [PubMed]
- Ng, L.-K.; Mulvey, M.R.; Martin, I.; Peters, G.A.; Johnson, W. Genetic characterization of antimicrobial resistance in Canadian isolates of Salmonella serovar Typhimurium DT104. Antimicrob. Agents Chemother. 1999, 43, 3018–3021. [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fifth Informational Supplement; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2015; ISBN 1-56238-989-0.



United States






