INTRODUCTION
Antimicrobial resistance is a major threat to human health (1). Host-directed therapy has emerged as a promising antibiotic-free strategy for disease control and prevention (2, 3). Host defense peptides (HDPs), also known as antimicrobial peptides, are small molecules of the innate immune system featuring antimicrobial and immunomodulatory properties (4, 5). Inducing HDP synthesis is a host-directed antimicrobial therapy that is being actively explored for human and livestock applications (2, 6, 7). HDPs are classified into two major families, namely defensins and cathelicidins, in vertebrate animals (4, 5). Defensins contain characteristic disulfide bridges and six cysteine residues that categorize them as α-, β-, or θ-defensins (8), while cathelicidins are identified by the presence of a conserved cathelin precursor (9). The chicken genome encodes a total of 14 β-defensins known as avian β-defensin 1–14 (AvBD1–14) and four cathelicidins known as CATH1-3 and CATHB1, with their expression detected throughout the gastrointestinal (GI), respiratory, reproductive, and urogenital tracts as well as in the bone marrow and several types of immune cells (10, 11).
Butyrate is a short-chain fatty acid produced primarily by bacterial fermentation in the GI tract (12, 13). Butyrate induces HDPs mainly by acting as a histone deacetylase inhibitor (HDACi) to enhance acetylation of histones, relaxation of gene promoters, and transcription of target genes (14). Forskolin (FSK) is a natural product belonging to the diterpene family extracted from the roots of Coleus forskohlii, a member of the mint family grown in India, Nepal, and Thailand that is traditionally used to treat various inflammation-related disorders (15). FSK acts as a natural adenylyl cyclase agonist to increase the synthesis of cyclic adenosine monophosphate (cAMP) (15), which in turn activates protein kinase A (PKA) resulting in phosphorylation and activation of a transcription factor known as cAMP responsive element (CRE)-binding protein (CREB) (16). Activated CREB then binds to a CRE region of its target gene promoter leading to increased gene transcription. FSK-mediated HDP gene induction is believed to transduce through the cAMPPKA signaling pathway (17, 18). It is well-known that a negative feedback loop exists for the cAMP-PKA pathway through upregulation of inducible cAMP early repressors (ICER) that represent a group of smaller proteins transcribed alternatively from the CRE modulator (CREM) gene (19). ICER suppresses gene transcription targeted by the cAMP-PKA-CREB pathway by competing with CREB for the CRE region of target gene promoters (19).
Integrity of mucosal barrier function is critical for the host to achieve homeostasis and defend against external insults from the environment (20). Barrier function is maintained mainly by the mucus layer and tight junctions (20). Mucin-2 (MUC2) constitutes a major component of the mucus in the GI tract (21), while tight junctions are formed by a network of transmembrane and cytosolic adaptor proteins such as claudin-1 (CLDN1) and zonula occludens-1 (ZO1) that join together the cytoskeletons of neighboring cells (22). Butyrate is capable of enhancing barrier function (12, 13), but the role of FSK in barrier function remains unknown.
We previously found butyrate and FSK to be capable of inducing chicken AvBD9 gene expression in a synergistic manner (18). In this study, we continued to investigate the role of butyrate and FSK in the expressions of other chicken HDPs both in vitro and in vivo. We also examined their impact on barrier function. Necrotic enteritis (NE), caused by Clostridium perfringens, is an economically devastating enteric disease and often associated with growth retardation, reduced feed efficiency, small intestinal pathology, and 10–20% mortality in chickens (23). The efficacy of dietary supplementation of butyrate and FSK in protecting chickens from NE was evaluated. The mechanism of action of the HDP-inducing synergy between butyrate and FSK was further explored using RNA sequencing and a chicken specific kinome peptide array. We revealed that butyrate and FSK synergistically enhanced innate host defense, barrier function, and disease resistance without triggering inflammation and that ICER is a major negative regulator of butyrate- and FSK-induced HDP gene expression.
MATERIALS AND METHODS
Cell Culture and Stimulation
Chicken HD11 macrophage cells (24) were maintained in Roswell Park Memorial Institute (RPMI) 1640 (Hyclone, Logan, UT) supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA). After overnight seeding in 12-well plates at 1 × 106 cells/well, HD11 cells were stimulated with different concentrations of sodium butyrate (MilliporeSigma, St. Louis, MO) and FSK (Santa Cruz Biotechnology, Santa Cruz, CA) individually or in combination. Cells were subjected to total RNA isolation for subsequent quantitative reverse transcription PCR (RT-qPCR) or RNA sequencing as described below.
Dietary Supplementation of Butyrate and FSK to Chickens
A total of 60 day-old male broiler chickens were obtained from Cobb-Vantress Hatchery (Siloam Springs, AR), divided randomly into six groups of 10, and provided ad libitum with tap water and a non-medicated commercial basal diet (DuMOR Chick Starter/Grower 20%, SKU #507821099, Tractor Supply Co., Brentwood, TN). Animals were raised in santitized floor pens with fresh pine wood shavings under standard management for 4 days. While one group of animals were allowed to continue on the basal diet, five other groups were switched to experimental diets supplemented with 1 g/kg microencapsulated sodium butyrate (CM3000® containing 30% pure sodium butyrate, King Techina, Hangzhou, China), 20 mg/kg C. forskohlii (CF) extract containing 10% FSK (Vitacost, Lexington, NC), or a combination of 1 g/kg CM3000® and CF extract (5, 10, or 20 mg/kg feed). After 2 days of feeding, eight chickens from each group were euthanized for collection of the jejunum at −80°C until homogenization for RNA extraction. All animal procedures were approved by the Institutional Animal Care and Use Committee of Oklahoma State University under protocol AG1610.
RT-qPCR Analysis of Gene Expression
Total RNA was isolated from chicken HD11 cells or the jejunal segments using RNAzol RT (Molecular Research Center, Cincinnati, OH) according to the manufacturer’s protocol. RNA was quantified using Nanodrop 1000 (Nanodrop Products, Wilmington, DE) and the ratios of A260/A230 and A260/A280 were used to indicate RNA quality. Reverse transcription was performed using Maxima First Strand cDNA Synthesis Kit (ThermoFisher Scientific, Pittsburgh, PA), followed by qPCR analysis with QuantiTect SYBR Green PCR Kit (Qiagen, Valencia, CA) in 10-µL reactions. The qPCR protocol consisted of an initial activation at 95°C for 10 min and 40 cycles of 94°C for 15 s, 55°C for 20 s, and 72°C for 30 s. Melting curve analysis was performed to determine PCR specificity, and relative fold changes were estimated with the ΔΔCt method (25) using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the reference gene for data normalization as we previously described (18, 26).
Chicken Model of Necrotic Enteritis
A total of 48 male day-of-hatch Cobb broiler chicks were obtained from Cobb-Vantress Hatchery and randomly assigned to four floor pens with 12 animals per pen. Birds were raised for 10 days on a non-medicated basal diet (DuMOR Chick Starter/Grower 20%, Tractor Supply Co.) under standard management. On day 10, chicks were weighed individually and 36 of similar body weights were transferred to battery cages with three cages per treatment and three animals per cage. Upon transfer, one group of animals continued with the basal DuMOR diet, while the other three groups were supplemented with 1 g/kg microencapsulated sodium butyrate (CM3000®, King Techina), 5 mg/kg CF extract (Vitacost), or a combination of 1 g/kg CM3000® and 5 mg/kg CF extract. Following a 3- day feeding and acclimatization, chickens were fasted overnight prior to daily challenges with the netB- and tpeL-positive C. perfringens strain Brenda B (provided kindly by Dr. Lisa Bielke at the Ohio State University) (27) to induce NE as described (28). C. perfringens was cultured sequentially in cooked meat medium (ThermoFisher Scientific) and fluid thioglycollate (ThermoFisher Scientific) broth every 18 h. Bacterial challenge was performed by feeding a 1:1 mixture of bacterial overnight culture and respective diets twice daily for 4 days. On day 19, all animals were euthanized by CO2 asphyxiation and necropsied to determine the duodenal and jejunal lesion scores in a blind manner according to a 6-point scoring system as described (29). A segment of the jejunum was also fixed in formalin (VWR International, Radnor, PA), processed, and stained with hematoxylin and eosin (VWR International). Additionally, the jejunal digesta was collected and the C. perfringens titer was determined using qPCR as described below.
Quantification of C. perfringens
Microbial DNA was isolated from the jejunal digesta using the ZR Fecal DNA Miniprep Kit (Zymo Research, Irvine, CA). DNA quality and quantity were determined using Nanodrop 1000. The C. perfringens titer was determined using a standard curvebased qPCR and C. perfringens-specific primers as described (30, 31). The standard curve was constructed using 10-fold serial dilutions of genomic DNA isolated from a known count of pure C. perfringens culture. The qPCR protocol consisted of an initial activation at 95°C for 10 min, followed by 40 cycles of 94°C for 15 s, 60°C for 20 s, and 72°C for 30 s. Melting curve analysis was performed to determine PCR specificity and the C. perfringens titer was calculated as colony-forming units (CFU)/g of the jejunal digesta.
Western Blot Analysis
Chicken HD11 cells were treated in duplicate with butyrate (2 mM) or FSK (5 or 200µM) individually or in two different combinations for 6 h. Cells were then lysed in 0.5 mL of TNT buffer (20 mm Tris, pH 7.5, 200 mm NaCl, 1% Triton X-100) containing a cocktail of protease inhibitors (MilliporeSigma). Proteins (50 µg) in the supernatants were separated on 12% SDS-PAGE, transferred to an ImmobilonP® polyvinylidene difluoride membrane (MilliporeSigma), and blotted sequentially with a rabbit anti-CREM/ICER polyclonal antibody (MilliporeSigma, #AV34777; diluted 1:500) and a goat anti-rabbit IgG secondary antibody conjugated with horseradish peroxidase (MilliporeSigma; diluted 1:2,000). The reactive bands were visualized using enhanced chemiluminescence (ThermoFisher Scientific).
RNA Sequencing and Analysis
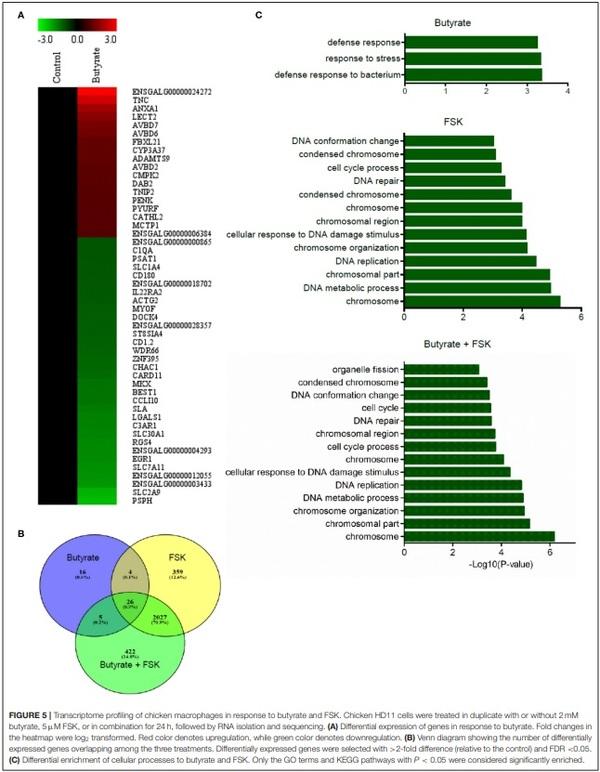
Chicken HD11 cells were treated in duplicate with or without 2 mM butyrate, 5µM FSK, or in combination for 24 h. Total RNA was isolated for cDNA library preparation using TruSeq RNA Sample Prep Kit (Illumina, San Diego, CA), followed by RNA sequencing by Novogene (Beijing, China) on an Illumina HiSeq 2000 platform. The raw sequence reads were filtered using Cutadapt (32) and Prinseq (33). High-quality clean reads from each sample were separately aligned to the chicken genome assembly (Ensembl, Gallus gallus 5.0) using TopHat2 (34). The resulting transcript abundances were quantified using a software package, RNA-Seq by Expectation Maximization (RSEM) (35). The expression levels were determined based on the values of fragments per kilobases per million mapped reads (FPKM). Differentially expressed genes with > 2-fold difference (relative to the control) and false discovery rate (FDR) < 0.05 were identified using edgeR (36) and visualized on a heat map using MultiExperiment Viewer (MeV) (37). To identify enriched gene ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, functional enrichment of the genes was analyzed using a web-based tool, KEGG OrthologyBased Annotation System, intelligent version (KOBAS-i) (38). Only the GO terms and KEGG pathways with P < 0.05 were considered significantly enriched. Venn diagram was generated using Venny 2.0 online software (https://bioinfogp.cnb.csic.es/ tools/venny/index.html).
Kinome Peptide Array and Analysis
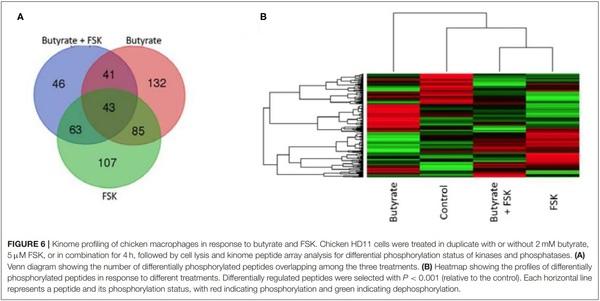
Chicken HD11 cells were treated in duplicate with or without 2 mM butyrate, 5µM FSK, or in combination for 4 h. Cell pellets were lysed using 100 µL lysis buffer (20 mM Tris– HCl pH 7.5, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM ethylene glycol tetraacetic acid (EGTA), 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM Na3VO4, 1 mM NaF, 1µg/mL leupeptin, 1 g/mL aprotinin, and 1 mM phenylmethylsulphonyl fluoride; all purchased from MilliporeSigma, St. Louis, MO). The supernatants were subsequently subjected to a chicken-specific kinome peptide array procedure as described (39) with slight modifications (40). The kinome peptide array was constructed to contain a total of 771 chicken-specific peptides that were derived from phosphorylation sites of 572 proteins. Data normalization and clustering analysis were performed as described (41). GO terms and KEGG pathway analysis were performed by uploading a list of statistically significant peptides (P < 0.001) to the Search Tool for the Retrieval of Interacting Genes (STRING) (42).
Statistical Analysis
For cell culture experiments, the results were shown as means ± standard error of the mean (SEM) of 3–4 independent experiments. Following the confirmation of normality of the data with Shapiro-Wilk test, statistical significance was determined using one-way ANOVA with post-hoc Tukey’s test in Prism (GraphPad Software, La Jolla, CA). Statistical significance was considered if P < 0.05. Treatments with P < 0.20 were also indicated to show a trend.
RESULTS
Butyrate and FSK Synergistically Induce Chicken HDP and Barrier Function Genes
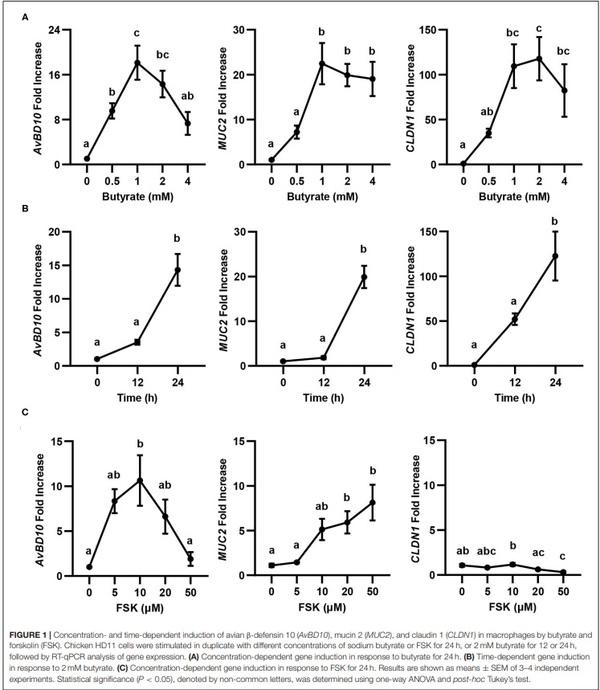
Butyrate is known to synergize with FSK in inducing chicken AvBD9 gene expression (18). To study whether the synergy also occurs with other HDP genes as well as two major genes involved in barrier function (MUC2 and CLDN1) (20), we first conducted both dose-response and time-course experiments in chicken HD11 macrophage cells with butyrate and FSK separately, followed by RT-qPCR analysis of gene expression. Similar to AvBD9 (18), AvBD10, MUC2, and CLDN1 were all induced by butyrate in both concentration- (Figure 1A) and time-dependent manners (Figure 1B). A peak induction of all three genes was observed with 1 mM butyrate at 24 h. A longer duration was not attempted because of much reduced cell numbers and hence RNA concentrations. Besides CLDN1, two other tight junction protein genes tested (CLDN5 and ZO1) were also significantly induced by butyrate (data not shown). Notably, higher concentrations of butyrate led to diminished inductions of all three genes examined (Figure 1A), presumably due to the presence of a negative feedback mechanism. FSK also showed a dose-dependent induction of AvBD10 and MUC2, but not CLDN1 (Figure 1C). Similar to AvBD9 (18), a maximum induction of AvBD10 by FSK occurred at 10µM and higher concentrations showed a reduced response, while 50µM FSK gave the strongest upregulation of MUC2 gene expression (Figure 1C). CLND1 failed to be induced by FSK at any concentrations tested, and was in fact significantly suppressed by 50µM FSK (Figure 1C). Time-course experiments with FSK were not attempted because 24 h was known to be optimal in giving a peak induction of AvBD9 (18).
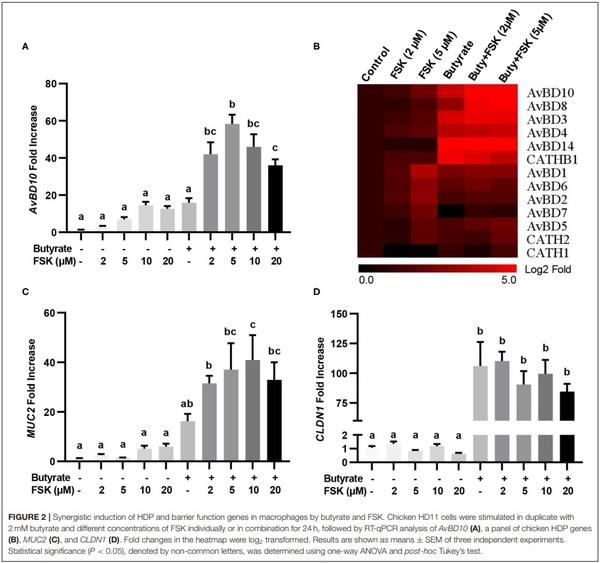
The synergy between butyrate and FSK in regulating HDP and barrier function genes was further explored. Similar to AvBD9 (18), AvBD10 was also induced by butyrate and FSK in a synergistic manner. A peak 58-fold induction of AvBD10 occurred in HD11 cells when 5µM FSK was combined with 2 mM butyrate, while only 7- and 16-fold increases were observed when FSK and butyrate were applied separately (Figure 2A). Additionally, AvBD3 and AvBD8 were synergistically induced in response to butyrate and FSK (Figure 2B). Although all other chicken HDP genes were induced to different magnitudes in response to butyrate or FSK, no clear synergy was observed (Figure 2B). It is noteworthy that approximately a half number of HDP genes were more responsive to butyrate, while the other half were more readily induced by FSK (Figure 2B). Desirably, MUC2 gene expression was synergistically enhanced by butyrate and FSK (Figure 2C), but no synergy was seen with CLDN1 (Figure 2D).
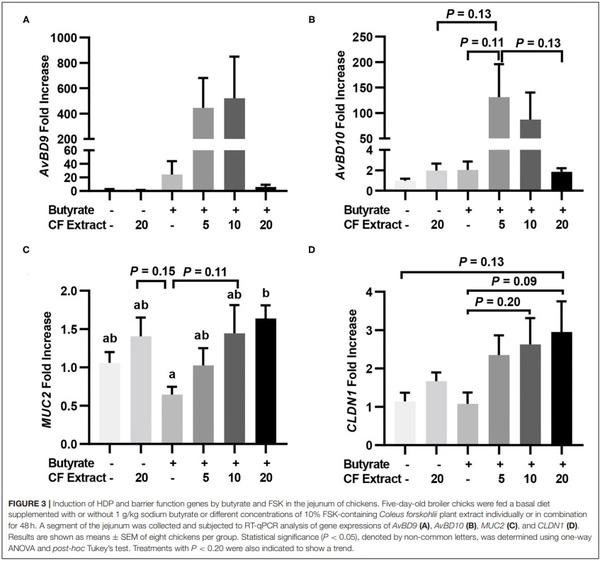
To verify whether HDP and barrier function gene induction would occur in vivo in response to butyrate and FSK, chickens were supplemented with 1 g/kg microencapsulated sodium butyrate or different concentrations of a 10% FSK-containing CF extract individually or in combination for 2 days before jejunal segments were harvested for RT-qPCR analysis of HDP and barrier function gene expression. CF extract at 20 mg/kg gave no or a minimum gene induction, while butyrate gave a modest 25- and 2-fold increase in AvBD9 and AvBD10, respectively (Figures 3A,B). Importantly, a combination of butyrate and 5 mg/kg CF extract led to an average 445- and 131-fold increase in AvBD9 and AvBD10, respectively (Figures 3A,B). MUC2 and CLDN1 expressions also tended to increase in the chicken jejunum in response to a combination of butyrate and FSK (Figures 3C,D).
Butyrate and FSK Synergistically Alleviate NE in Chickens
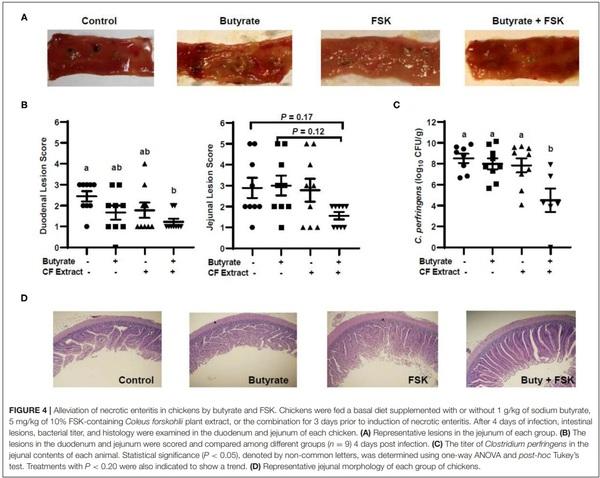
To evaluate the efficacy of butyrate and FSK in alleviating NE, chickens were supplemented with butyrate and FSK-containing CF extract individually or in combination for 4 days prior to daily challenges with C. perfringens to induce NE as described (28). As expected, the C. perfringens infection developed characteristic gross lesions in the small intestine (Figure 4A). Average lesion scores of 2.5 and 3 were observed in the duodenum and jejunum, respectively, if left unintervened (Figure 4B). Butyrate or FSK at the concentrations used tended to reduce the lesions in the duodenum, but not in the jejunum. Importantly, a combination of butyrate and FSK caused a significant decrease in the lesion score in the duodenum (P < 0.05) with a strong tendency also in the jejunum (P = 0.17) (Figure 4B). Similarly, butyrate and FSK alone failed to suppress C. perfringens colonization in the jejunum, and only the combination gave a significant, 4-log reduction in the bacterial titer (P < 0.05) (Figure 4C). Consistently, C. perfringens infection gave an obvious damage on the jejunal mucosa, and supplementation of butyrate or FSK alone showed no visible improvement, while only the combination was capable of restoring the epithelial integrity (Figure 4D). Overall, these results indicated a strong synergy between butyrate and FSK in ameliorating NE in broiler chickens.
Butyrate and FSK Enhance Barrier Function Without Triggering Inflammation
To achieve a global understanding of the effect of butyrate and FSK on the transcriptome, RNA sequencing was performed with chicken HD11 cells following a 24-h treatment with or without butyrate, FSK or in combination. A total of 51 genes were found to be differentially regulated by butyrate, with 18 genes being upregulated and 33 downregulated (fold change > 2 and FDR < 0.05) (Figure 5A). Of note, four chicken HDP genes including AvBD2, AvBD6, AvBD7, and CATH2, were upregulated. FSK differentially regulated 2,416 genes, while the butyrate/FSK combination resulted in differential regulation of 2,480 genes (Figure 5B). Of all differentially regulated genes, a majority (2, 27) were regulated by both FSK and the butyrate/FSK combination, while 422 genes were uniquely regulated by the combination. Further evaluation of the cellular processes revealed that butyrate preferentially impacted defense and stress responses, while most processes affected by FSK and the butyrate/FSK combination were involved in DNA replication and repair and chromosomal organization (Figure 5C).
To further understand a possible involvement of different signaling pathways in butyrate- and FSK-mediated response, HD11 cells were stimulated with butyrate and FSK individually or in combination for 4 h and then subjected to a chicken specific kinome peptide array analysis as described (41). Among a total of 771 peptides analyzed, 301, 298, and 193 peptides were differentially phosphorylated by butyrate, FSK, and the combination, respectively (P < 0.001) (Figure 6A). Of those, 107 peptides were differentially phosphorylated only by the butyrate/FSK combination. A unique phosphorylation pattern was observed with each treatment, with FSK and the butyrate/FSK combination more resembling each other (Figure 6B). STRING analysis (42) further revealed that 116 and 100 pathways were significantly affected by butyrate and FSK, respectively, while 89 pathways were significantly altered by the combination (Supplementary Figure 1).
Of all genes and pathways regulated by a combination of butyrate and FSK, a special attention was given to those involved in barrier function and inflammation, given their significance in intestinal health. RNA sequencing revealed that several genes involved in barrier function such as CLDN1 and PAR6 were significantly induced by butyrate (FDR < 0.05), while FSK caused a significant reduction in CLDN1 expression with a minimum impact on other tight junction genes (Supplementary Figure 2). A concomitant treatment with butyrate and FSK restored the beneficial effect of butyrate showing significant upregulation of several barrier function genes such as CLDN1 CLDN5, CLDN10, PAR6, and MUC5B (FDR < 0.05) (Supplementary Figure 2). Similarly, kinome peptide array showed that both butyrate and the butyrate/FSK combination induced a significant phosphorylation of multiple proteins involved in tight junction assembly such as Wiskott– Aldrich syndrome protein (WASP), RhoA, and Rho kinase (ROCK) (P < 0.001) (Supplementary Figure 3). These results suggested a beneficial role of butyrate and butyrate/FSK in enhancing barrier function. However, it is noted that junctional adhesion molecule 2 (JAM2) and JAM3 were significantly downregulated by both butyrate and the butyrate/FSK combination (Supplementary Figure 2), and both proteins were dephosphorylated by butyrate, but restored in response to the butyrate/FSK combination (Supplementary Figure 3).
Butyrate and FSK are known to be anti-inflammatory (12, 43). As expected, no pro- or antiinflammatory genes were significantly affected by butyrate, while interleukin-8 (IL-8), IL-10, and IL-19 were induced by FSK, and IL-8, IL-19, and interferon-β (IFN-β) were augmented by the butyrate/FSK combination (data not shown). IL-1β and IFN-γ were not induced by either compound or their combination. Consistently, nuclear factor-κB (NF-κB) signaling pathway, a major pathway involved in inflammatory response (44), was not significantly affected by butyrate and FSK in either RNA sequencing or kinome peptide array analysis.
ICER Is Induced by Butyrate and FSK and Suppresses HDP Gene Induction
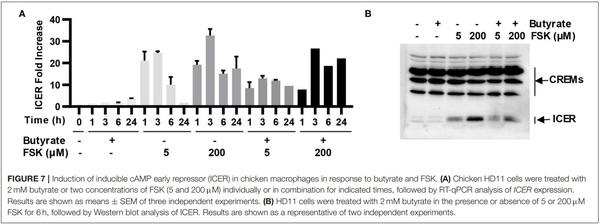
ICER is a major suppressor of the cAMP-PKA-CREB pathway (19), which can otherwise be activated by FSK (15). To study whether ICER is involved in reduced synergy between butyrate and higher concentrations of FSK observed earlier in both cell culture (Figure 2A) and live animals (Figures 3A,B), HD11 cells were treated for various times with 2 mM butyrate and two different concentrations of FSK (5 and 200µM) individually or in combination. It was apparent that both concentrations of FSK quickly induced ICER mRNA expression, with a peak 25-fold induction at 3 h. However, 200µM FSK increased ICER transcription more strongly with a 32-fold upregulation at 3 h, and the induction was maintained at 18-fold even at 24 h, whereas the ICER expression was largely reduced to a basal level at 24 h in response to 5µM FSK (Figure 7A). In contrast, ICER was gradually upregulated in response to butyrate in a time-dependent manner, with a minimum induction at 1–3 h and peaking at 24 h. ICER mRNA expression was maximum at 3 h, but remained substantially elevated at 24 in response to a combination of butyrate and FSK, with 200µM FSK sustaining the ICER transcription by 21-fold at 24 h (Figure 7A). At the protein level, 200µM FSK induced ICER synthesis at a much higher level than 5µM FSK with or without butyrate (Figure 7B). These results suggested a reduced synergy between butyrate and higher concentrations of FSK observed earlier was likely due to a stronger induction of ICER.
DISCUSSION
In this study, we have demonstrated two natural products, butyrate and FSK, showing a synergistic activity in enhancing HDP genes as well as several major genes involved in barrier function without triggering inflammatory response. Furthermore, dietary supplementation of butyrate and FSK are synergistically protective against NE, a devastating enteric disease that costs $6 billion annually to the global poultry industry (23). The HDP-inducing, barrier-protective, and anti-inflammatory properties of butyrate and FSK have been confirmed independently by qPCR, RNA sequencing, and kinome peptide array. For example, both qPCR and RNA sequencing have demonstrated an induction of multiple, but not all, HDP genes in response to butyrate and FSK. Differential response of HDP genes is likely reflected by close proximity of those genes. For example, AvBD8, AvBD9, and AvBD10 are located in tandem on chicken chromosome 3 (45) and synergistically induced by butyrate and FSK. It is conceivable that these three HDP genes are likely originated from gene duplication and thus regulated similarly. It is interesting to note that approximately a half number of chicken HDP genes are highly responsive to butyrate, while the other half are responsive to FSK. Those FSK-responsive AvBD genes (AvBD1/2/5/6/7) are largely located continuously in one gene cluster on chromosome 3 that interrupts two clusters of butyrate-responsive genes (AvBD8/19/10 and AvBD3/4/14) (45, 46). Another gene cluster (AvBD11/12/13) are barely detectable in HD11 cells and therefore, no results were shown for them. Among chicken cathelicidins, CATHB1 is readily induced by butyrate, while CATH1 and CATH2 are more regulated by FSK. No synergy has been observed with any of those FSK-responsive HDP genes, while a half number of butyrate-responsive genes are induced by butyrate and FSK in a synergistic manner.
It is noted that nearly all chicken HDP genes are induced in HD11 cells in response to butyrate and FSK by RTqPCR; however, RNA sequencing has only revealed significant upregulation of four (AvBD2, AvBD6, AvBD7, and CATH2). This apparent discrepancy is likely due to different sensitivities of the two technologies. RNA sequencing may not be sensitive enough to reliably detect those genes that are expressed in HD11 cells at extremely low levels. Furthermore, the threshold (FDR < 0.05 and fold change > 2) may be too stringent for a few HDP genes to be selected as differentially expressed genes. Nevertheless, both RT-qPCR and RNA sequencing highlight desirable preferential induction of multiple HDPs in response to butyrate and FSK.
Integrity of mucosal barrier function is maintained by the mucus layer and tight junctions (20). Butyrate is known to improve barrier integrity by inducing the expressions of MUC2 and multiple proteins involved in tight junction assembly (12). In our study, qPCR confirmed induction of MUC2, CLDN1, and CLDN5 by butyrate, and we showed for the first time that FSK is also capable of inducing MUC2, a predominant component of the mucus layer in the GI tract (21). Consistently, we demonstrated that butyrate and FSK cooperatively enhance MUC2 expression both in vitro and in vivo. Although FSK suppresses CLDN1 gene expression, a combination of butyrate and FSK reverses such an effect resulting in an upregulation of multiple claudins such as CLDN1, CLDN5, and CLDN10 as revealed by RNA sequencing. Kinome peptide array further revealed butyrate- and FSK-mediated phosphorylation of many proteins that are involved in the assembly of tight junctions such as ROCK and WASP. ROCK is a serine-threonine kinase that regulates phosphorylation of myosin light chain and F-actin, thus affecting actin polymerization and cell contraction and tight junction assembly (47). Phosphorylation and activation of ROCK also activates p21-activated kinase, which in turn activates WASP (48), resulting in the nucleation and polymerization of actin filaments and reduced permeability of tight junctions (49).
Both butyrate and FKS have been shown to be primarily anti-inflammatory (12, 43). Previous work in chicken HD11 cells has found butyrate to have no effect on IL-1β expression and a minimum effect on IL-6, IL-8, and IL-12p40 (50, 51). Similarly, FSK showed no induction of IL-1β, while a combination of butyrate and FSK showed a minimum increase in IL-1β expression (data not shown). Consistently, RNA sequencing showed no significant induction of IL-1β or many other typical proinflammatory cytokine genes in response to butyrate and FSK. Furthermore, kinome peptide array indicated a minimum effect on the NF-κB pathway as well.
Butyrate is known to induce HDP expression mainly by functioning as HDACi to cause hyperacetylation of histones and hence chromatin relaxation (14). Consequently, a number of HDACi have been identified to enhance HDP expression (52–54). FSK is a natural agonist of adenylyl cyclase to activate the cAMP-PKA-CREB signaling pathway (15), which is involved in FSK-mediated HDP induction (17, 18). However, the mechanism underlying butyrate- and FSK-mediated synergy in HDP induction remains largely unknown, although both the mitogen-activated protein kinase and the cAMP-PKA pathways are involved (18).
Our preliminary studies indicated that neither FSK causes histone acetylation in HD11 cells, nor the butyrate/FSK combination leads to increased acetylation of histones relative to butyrate alone (data not shown), suggesting that histone hyperacetylation is not involved in HDP-inducing synergy between butyrate and FSK. However, biological processes such as DNA repair and chromosome organization are predominantly enriched in FSK- and butyrate/FSK-treated HD11 cells as revealed by RNA sequencing, suggesting that chromatin remodeling might be involved. Consistently, activation of the cAMP-PKA signaling was recently found to induce DNA damage and suppress DNA repair (55, 56), which subsequently help relieve DNA torsional stress and activate gene transcription at DNA damage sites, while global transcription is transiently silenced (57–59). Perhaps unsurprisingly, several small-molecule compounds that are inducers of DNA damage were recently found to upregulate HDP gene expression in a high throughput screening (53).
Furthermore, we have revealed a negative feedback mechanism involved in diminished HDP induction in response to higher concentrations of FSK with or without butyrate. ICER is transcriptionally induced by activation of the cAMP-PKACREB signaling pathway and serves as a major negative feedback mechanism to suppress gene transcription by competing with CREB for the same CRE region of the target gene promoter (19). We have found that ICER is quickly induced within 1 h, peaked at 3 h, and greatly subdued at 24 h in response to FSK or the FSK/butyrate combination, but higher concentrations of FSK keep ICER expression elevated even after 24 h, which is presumably responsible for reduced HDP gene expression.
CONCLUSIONS
In summary, we have demonstrated a synergy in the induction of multiple, but not all, HDP genes between butyrate and FSK both in vitro and in vivo. Barrier function is also positively impacted. Desirably, butyrate and FSK enhance HDP expression and barrier integrity without causing inflammation. Consistently, butyrate and FSK are capable of ameliorating NE in a synergistic fashion. Therefore, butyrate and FSK, two natural products with HDP-inducing, barrier protective, and anti-inflammatory activities, have the potential to be developed as novel antibiotic alternatives for disease control and prevention in poultry and possibly other animals.



















