Bacteriophage therapy in effective control of E.Coli infections in poultry
Published: August 5, 2019
By: Dr. Ajay Chalikwar / Zonal Technical Manager, Provet Pharma PVT. LTD. Chennai, India.
INTRODUCTION
- The poultry market in India is a fast growing and demanding market.
- India's poultry industry has transformed from a mere backyard activity into a major commercial activity in just four decades.
- India is now the world's 4th largest egg producer and the 5th largest producer of broilers.
- The Indian poultry market witnessed an increasing trend over the past five years, growing at a compound annual growth rate of 11.39%.
- India's per capita consumption of the poultry meat is estimated at around 3.1kg/year, which is low compared to the world average of around 17 kg per year.
- The per capita availability of egg is 69/year in India which is also low compared to the world average 179/year. (Source: Poultry entrepreneurship programme 2019)
- This is putting tremendous pressure on birds in terms of weight gain, feed conversion efficiency, disease challenge and antimicrobials’ resistance.
- Major challenges are bacterial, viral, metabolic, mycotoxins, etc.
- E.Coli is emerging as major infections in broilers, layers and breeders and irrespective of good water sanitation programme it is commonly encountered in almost all the poultry operations at some time period including hatchery, meat processing plant, etc.
- Let’s discuss regarding E.Coli infections in poultry and its effective control through use of non-conventional therapy like BACTERIOPHAGE.
BRIEF ABOUT E.Coli
- E.Coliis a Gram-negative, optional anaerobic, rod-shaped, non-spore forming, coliform bacterium belongs to Enterobacteriaceae.
- These strains of E.Coli falls under APEC (Avian pathogenic E.Coli) and most of the strain found in poultry are extra-intestinal.
- In poultry, mostly systemic diseases are seen unlike mammals, where gut related diseases are commonly seen with E.Coli.
- Most strains are motile having peritrichous flagella.
- The organism survives freezing and persists for extended periods at cold temperatures (1).
- Reproduction of most strains is inhibited by a pH of less than 4.5 or greater than 9, but the organism is not killed.
- It is commonly seen as secondary infection to other bacterial/viral infections or sometimes as primary infectious agent but recent trends shows that they have ability to cause disease on their own.
- It is a typically localized or systemic infection when host defences have been impaired.
- Most frequent cause of mortality.
- Even can be isolated from dead birds.
- Normally poultry house dust contains 105 to 106 CFU/gm of E.Coli inside the houses and outside up to 40 feet area under dry conditions but wetting reduces the count by 84-97%.
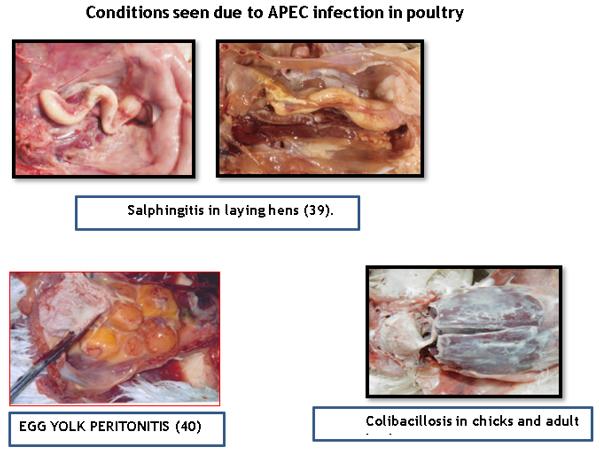
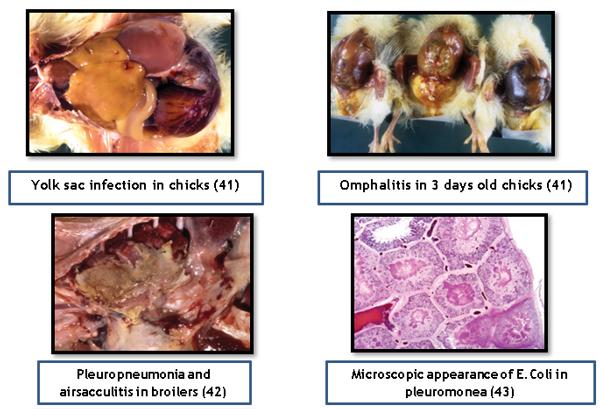
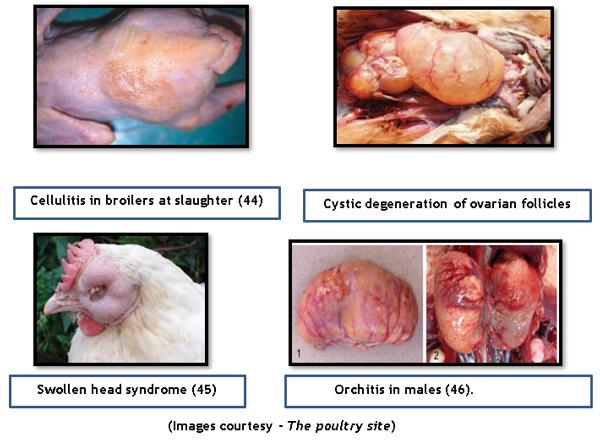
- E.Coli produce variety of diseases in poultry like Omphalitis, salphingitis, egg yolk peritonitis, colisepticemia, Colibacillosis, cystic degeneration of ovarian follicles, cellulitis, coligranuloma, arthritis, sternal bursitis, etc.
Economic impact of APEC is mainly due to
- Mortality
- Morbidity
- Slower growth rates
- Poor FCR
- Decrease in egg production
- Carcass downgrading
- Spreads antimicrobial resistance to other bacteria
Antigenic structures and toxins of E.Coli
- Antigens are classified according to Kauffmann scheme (2).
- It contains 3 major antigens like O (somatic), H (flagellar), K (capsular), F (Pilus).
- Currently, there are approximately 180-O, 60-H and 80-K antigens (3)
- The O antigen determines serogroup and addition of the H antigen and sometimes K antigen determines serotype (4).
- APEC doesn’t produce enterotoxins unlike mammalian species.
- Immune response in poultry is directed against O antigen (5).
- Common serotypes found are O1, 02, 018, 035, 036, 078, 0111 (6)
Virulence factors involved
- Adhesins- It may be fimbrial or non fimbrial (7), F1 fimbriae may be expressed during initial colonization whereas P fimbriae are expressed later when the organism is in the lower respiratory tract or body tissues.
- Bacteria are killed rapidly following expression of F1 fimbriae by macrophages (8).
- Iron acquisition methods- Ability of APEC to obtain iron is well documented (9).
- Protectins – Resistance to APEC to complement is related to K1 capsule (10) , smooth LPS layer (11) , Outer membrane protein (12) , Increased serum survival gene (ISS) in APEC has its role in complement resistance with a CoIV plasmid which increase virulence in day old chicks by 100 times (13).
Factors that increase host susceptibility to E.Coli infection
- Virus- Adenovirus (14), Avian Influenza virus (15), Newcastle disease virus (16), Infectious Bronchitis virus (17), Avian Metapneumo virus (18), Infectious bursal disease (19), Marek’s disease virus (20), Chicken infectious anaemia virus (21).
- Bacteria – Mycoplasma Gallisepticum, Pasteurella Multocida, Staphylococcus sp., Streptococcus sp. (22).
- Others – Invasion of skin barrier (23), Excess ammonia in sheds (24), mycotoxins like Ochratoxin (25) and Fumonison (26), young age group (27), stress of various origin (28), sex- male are more prone (29), contaminated water and feed (30), dry dusty condition (31), feed/water restriction (30), parasitic infection (32), Intestinal Coccidiosis (33), Caecal coccidiosis (34), inadequate ventilation (35), dysbacteriosis, relatively high ammonia (36) overcrowding, poor litter condition, temperature extremes and high temperature and high humidity (1).
- Nutritional – Hypervitaminosis E (37), Hypervitaminosis A & Vitamin A deficiency (38), excess Iron & Selenium.
- Adult house flies acts as mechanical vectors and harbour organism in their body and cause horizontal transfer of antibiotic resistance and virulence genes.
- Low incubation period – 3-5 days.
Resistance of APEC (E.Coli) to heavy metals, disinfectants and antibiotics
- APEC has ability to acquire resistance to Chlorohexidine, Formaldehyde, hydrogen peroxide, and quaternary ammonium compounds through acquiring resistance often encoded by large R plasmids (47).
- APEC Inc H12 plasmid, PAPEC-O1-R plasmid confers resistance to silver, copper sulphate, Benzolkonium chloride following transfer of plasmid to recipient strain by conjugation (48).
- APEC Inc F IIA plasmid confers resistance to aminoglycosides, quaternary ammonium compounds, phenolic, β lactam antibiotics and silver (49).
- APEC Inc A/C plasmid confers resistance to aminoglycosides, sulpha drugs, quaternary ammonium compounds, phenolic, tetracycline and mercury (50).
- It has inherent ability to form biofilm on abiotic surfaces like plastic/metal waterlines (51) and protect itself from getting killed by the sanitizers.
- Certain plasmids may also contribute to APEC’s acid and bile tolerance, affecting survival of APEC in the bird or elsewhere (52)
- But there is some regional variations on certain strains of APEC as per as resistance is concerned, some strains are still susceptible (53)
E.Coli Control through use of bacteriophage
- Bacteriophage (54) is a non-pathogenic virus belonging to family with vast majority (about 96%) of known phages belongs to the Myoviridae, Podoviridae and Siphoviridae (55).
- They are known as bacterial parasites because they lack the cell structure and enzyme systems necessary for food uptake, protein synthesis or construction of new particles, and as incomplete, organisms can only replicate in a live cell (56).
- Positive results of the use of bacteriophages in fighting bacterial infections have contributed to the development of research on the potential use of viruses that destroy bacteria in treatment of diseases in both human and animals (57).
- Their specificity and range of activity is determined by the presence of receptors located on the surface of bacterial cells, among which we can distinguish LPS fragments, fimbriae and other surface proteins (58).
- No mortality was observed in chickens treated with 108 PFU of an E. coli bacteriophage mixture, another positive effect of the treatment was the absence of visible clinical symptoms (59).
- Due to the specificity of bacteriophages for particular bacteria, manifested as the ability to infect only one species, serotype or strain and this mechanism of action does not cause destruction of the commensal intestinal microflora.
- It should be cocktail of more than one phages (at-least 3-4) in sufficient concentration to get synergistic effect.
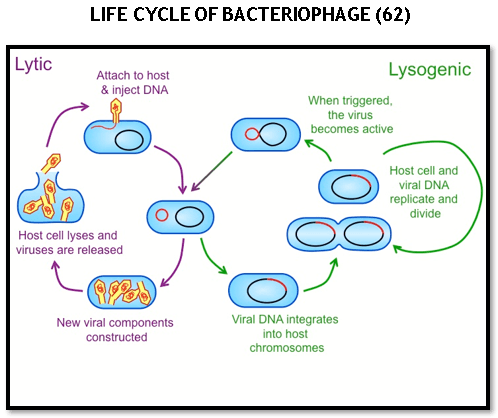
Bacteriophage has 2 life cycles
Lytic-
Here phage act like typical virus, it hijack its host cell and uses cell’s resources to make lots of new phages, thereby causing lysis of the cell and die subsequently.
In lytic phase the PHAGE will attach, enter inside the cell, copies DNA and synthesize protein, assembles new phages and cause lysis of the host cell and release of daughter phages capable of infecting other cells (60).
Lysogenic-
It allows a PHAGE to reproduce without killing its host.
Some phages can use the lytic life cycle but few phages can switch between the two cycles.
In lysogenic life cycle there will be attachment of phage to host cell, injecting DNA into host cell, integration with bacterial chromosome and becoming prophage and finally cell division along with host cell.
Under the right conditions, the prophage can become active and come back out of bacterial chromosome and triggering lytic life cycle and start killing host cell (61).
Advantage of cocktail of phages
- Phage 1 –The lead phages – It has a very virulent activity and neutralize a bacterial population within few hours of inoculation against wide spectrum of E.Coli in poultry.
- Phage 2 – The associate phage – It has lower virulence than lead phage but has very wide spectrum of activity and very stable, this phage can cover the bacterial population which invaded action of lead phage.
- Phage 3- The guard phage – It is similar to associate phage but it can attach to a different king of receptor and different mode of action in lytic action compared to lead and associate phage and it is mainly added to ensure protection against resistant E.Coli.
- Synergistic action of these 3 phages will ensure complete bactericidal action.
CONCLUSION
- Bacteriophage therapy is a very good solution as preventive measure in chick in first week itself and as therapeutic thereafter as per the infection pressure.
- Some authors suggest that similar effects of bacteriophage therapy like antibiotics will be seen preventing early development of colibacillosis in chicks (63).
- There are evidences that bacteriophages have the ability to penetrate the blood-brain barrier, and confirmed that the bacteriophages had a prophylactic effect in addition to the therapeutic effect (64).
- Bacteriophage can be given as orally, through aerosol spray or parenterally or along with antibiotics in case of severe E.Coli challenge (64).
- Bacteriophage can be given along with all other live and inactivated vaccines (except live E.Coli vaccine).
- Bacteriophage can enter in blood circulation upon oral administration and controls E.Coli systemically (64).
- Himathongkham, S., H. Riemann, S. Bahari, S. Nuanualsuwan, P. Kass, and D.O. Cliver. 2000. Survival of Salmonella typhimurium and Escherichia coli O157:H7 in poultry manure and manure slurry at sublethal temperatures. Avian Diseases. 44:853–860.
- Stenutz, R., A. Weintraub, and G. Widmalm. 2006. The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol Rev. 30:382–403.
- Kaper, J.B., J.P. Nataro, and H.L. Mobley. 2004. Pathogenic Escherichia coli. Nat Rev Microbiol. 2:123–140.
- Heller, E.D., and N. Drabkin. 1977. Some characteristics of pathogenic E. coli strains. Br Vet J. 133:572–578.
- Sharada, R., G. Krishnappa, and H.A. Upendra. 2001. Serological ‘O’ grouping and drug susceptibility of Escherichia coli strains from chicken. Indian Vet J. 78:78–79.
- Sojka, W.J. 1965. Escherichia coli in Domestic Animals and Poultry. Commonwealth Agricultural Bureau, Farnham Royal, England. 1–231.
- Babai, R., B.E. Stern, J. Hacker, and E.Z. Ron. 2000. New fi mbrial gene cluster of S-fimbrial adhesin family. Infection and Immunity. 68:5901–5907.
- Pourbakhsh, S.A., M. Boulianne, B. Martineau-Doize, and J. M. Fairbrother. 1997. Virulence mechanisms of avian fimbriated Escherichia coli in experimentally inoculated chickens. Veterinary Microbiology. 58:195–213.
- Caza, M., F. Lepine, and C.M. Dozois. 2011. Secretion, but not overall synthesis, of catecholate siderophores contributes to virulence of extraintestinal pathogenic Escherichia coli. Molecular Microbiology. 80:266–282.
- Cross, A.S., P. Gemski, J.C. Sadoff, F. Orskov, and I. Orskov. 1984. The importance of the K1 capsule in invasive infections caused by Escherichia coli. The Journal of Infectious Diseases. 149:184–193.
- Cross, A.S., J.C. Sadoff, P. Gemski, and K.S. Kim. 1988. The relative role of lipopolysaccharide and capsule in the virulence of E. coli. In: Bacteria, Complement and the Phagocytic Cell, Vol. H2., F. Cabello and C. Pruzzo, eds. Springer-Verlag, Berlin. 319–334.
- Binns, M.M., J. Mayden, and R.P. Levine. 1982. Further characterization of complement resistance conferred on Escherichia coli by the plasmid genes traT of R100 and iss of ColV,I-K94. Infection and Immunity. 35:654–659.
- Binns, M.M., D.L. Davies, and K.G. Hardy. 1979. Cloned fragments of the plasmid ColV,I-K94 specifying virulence and serum resistance. Nature. 279:778–781.
- Dhillon, A.S. 1986. Pathology of avian adenovirus serotypes in the presence of Escherichia coli in infectious-bursal-diseasevirus-infected specific-pathogen-free chickens. Avian Diseases. 30:81–86.
- Bano, S., K. Naeem, and S.A. Malik. 2003. Evaluation of pathogenic potential of avian influenza virus serotype H9N2 in chickens. Avian Diseases. 47:817–822.
- Huang, H.J., and M. Matsumoto. 2000. Nonspecific innate immunity against Escherichia coli infection in chickens induced by vaccine strains of Newcastle disease virus. Avian Diseases. 44:790–796.
- Cook, J.K.A., M.B. Huggins, and M.M. Ellis. 1991. Use of an infectious bronchitis virus and Escherichia coli model infection to assess the ability to vaccinate successfully against infectious bronchitis in the presence of maternally-derived immunity. Avian Pathology: Journal of the W.V.P.A. 20:619–626.
- Jirjis, F.F., S.L. Noll, D.A. Halvorson, K.V. Nagaraja, F. Martin, and D.P. Shaw. 2004. Effects of bacterial coinfection on the pathogenesis of avian pneumovirus infection in turkeys. Avian Diseases. 48:34–49.
- Nakamura, K., N. Yuasa, H. Abe, and M. Narita. 1990. Effect of infectious bursal disease virus on infections produced by Escherichia coli of high and low virulence in chickens. Avian Pathology: Journal of the W.V.P.A. 19:713–721.
- Friedman, A., E. Shalem Meilin, and E.D. Heller. 1992. Marek’s disease vaccines cause temporary B-lymphocyte dysfunction and reduced resistance to infection in chicks. Avian Pathology: Journal of the W.V.P.A. 21:621–631.
- Naqi, S., G. Thompson, B. Bauman, and H. Mohammed. 2001. The exacerbating effect of infectious bronchitis virus infection on the infectious bursal disease virus-induced suppression of opsonization by Escherichia coli antibody in chickens. Avian Diseases. 45:52–60.
- Murakami, S., M. Miyama, A. Ogawa, J. Shimada, and T. Nakane. 2002. Occurrence of conjunctivitis, sinusitis and upper region tracheitis in Japanese quail (Coturnix coturnix japonica), possibly caused by Mycoplasma gallisepticum accompanied by Cryptosporidium sp. infection. Avian Pathology: Journal of the W.V.P.A. 31:363–370.
- Hamdy, M.K., and N.D. Barton. 1966. Escherichia coli in normal and traumatized tissues. Proc Soc Exp Biol Med. 122:661–665.
- Nagaraja, K.V., D.A. Emery, K.A. Jordan, V. Sivanandan, J.A. Newman, and B.S. Pomeroy. 1984. Effect of ammonia on the quantitative clearance of Escherichia coli from lungs, air sacs, and livers of turkeys aerosol vaccinated against Escherichia coli. American Journal of Veterinary Research. 45:392–395.
- Kumar, A., N. Jindal, C.L. Shukla, R.K. Asrani, D.R. Ledoux, and G.E. Rottinghaus. 2004. Pathological changes in broiler chickens fed ochratoxin A and inoculated with Escherichia coli. Avian Pathology: Journal of the W.V.P.A. 33:413–417.
- Li, Y.C., D.R. Ledoux, A.J. Bermudez, K.L. Fritsche and G.E. Rottinghaus. 1999. Effects of fumonisin B1 on selected immune responses in broiler chicks. Poultry Science. 78:1275–1282.
- Goren, E. 1978. Observations on experimental infection of chicks with Escherichia coli. Avian Pathology: Journal of the W.V.P.A. 7:213–224.
- Huff, G.R., W.E. Huff, J.M. Balog, and N.C. Rath. 2001. Effect of early handling of turkey poults on later responses to multiple dexamethasone-Escherichia coli challenge. 2. Resistance to air sacculitis and turkey osteomyelitis complex. Poultry Science. 80:1314–1322.
- Huff, G.R., W.E. Huff, J.M. Balog, and N.C. Rath. 1999. Sex differences in the resistance of turkeys to Escherichia coli challenge after immunosuppression with dexamethasone. Poultry Science. 78:38–44.
- Harry, E.G. 1964. The survival of E. coli in the dust of poultry houses. The Veterinary Record. 76:466–470.
- Nagi, M.S., and L.G. Raggi. 1972. Importance to ‘airsac’ disease of water supplies contaminated with pathogenic Escherichia coli. Avian Diseases. 16:718–723
- Permin, A., J.P. Christensen, and M. Bisgaard. 2006. Consequences of concurrent Ascaridia galli and Escherichia coli infections in chickens. Acta Vet Scand. 47:43–54.
- Hein, H., and L. Timms. 1972. Bacterial flora in the alimentary tract of chickens infected with Eimeria brunetti and in chickens immunized with Eimeria maxima and cross-infected with Eimeria brunetti. Experimental Parasitology. 31:188–193.
- Koynarski, V., T. Mircheva, S. Stoev, V. Urumova, D. Zapryanova, E. Dishlyanova, T.S. Koynarski, and R.S. Karov. 2010. Pathoanatomical and blood biochemical investigations in chicks, challenged with Escherichia coli on the background of a pre-existing Eimeria infection. Revue Med Vet. 161:133–140.
- Nakamura, K., Y. Imada, and M. Maeda. 1986. Lymphocytic depletion of bursa of Fabricius and thymus in chickens inoculated with Escherichia coli. Vet Pathol. 23:712–717.
- Murakami, S., M. Miyama, A. Ogawa, J. Shimada, and T. Nakane. 2002. Occurrence of conjunctivitis, sinusitis and upper region tracheitis in Japanese quail (Coturnix coturnix japonica), possibly caused by Mycoplasma gallisepticum accompanied by Cryptosporidium sp. infection. Avian Pathology: Journal of the W.V.P.A. 31:363–370.
- Friedman, A., I. Bartov, and D. Sklan. 1998. Humoral immune response impairment following excess vitamin E nutrition in the chick and turkey. Poultry Science. 77:956–962.
- Friedman, A., A. Meidovsky, G. Leitner, and D. Sklan. 1991. Decreased resistance and immune response to Escherichia coli infection in chicks with low or high intakes of vitamin A. J Nutrition. 121:395–400.
- Bisgaard, M., and A. Dam. 1980. Salpingitis in poultry. I. Prevalence, bacteriology and possible pathogenesis in broilers. Nord Vet. 32:361–368.
- Trampel, D.W., Y. Wannemuehler, and L.K. Nolan. 2007. Characterization of Escherichia coli isolates from peritonitis lesions in commercial laying hens. Avian Diseases. 51:840–844.
- Harry, E.G. 1957. The effect on embyonic and chick mortality of yolk contaminated with bacteria from the hen. The Veterinary Record. 69:1433–1439.
- Nakamura, K., K. Imai, and N. Tanimura. 1996. Comparison of the effects of infectious bronchitis and infectious laryngotracheitis on the chicken respiratory tract. J Comp Pathol. 114:11–21.
- Cheville, N.F., and L.H. Arp. 1978. Comparative pathologic findings of Escherichia coli infection in birds. J Am Vet Med Assoc. 173:584–587.
- Aarestrup, F.M., and H. Hasman. 2004. Susceptibility of different bacterial species isolated from food animals to copper sulphate, zinc chloride and antimicrobial substances used for disinfection. Veterinary Microbiology. 100:83–89.
- Morley, A.J., and D.K. Thomson. 1984. Swollen-head syndrome in broiler chickens. Avian Diseases. 28:238–243.
- Monleon, R., M.P. Martin, and H.J. Barnes. 2008. Bacterial orchitis and epididymo-orchitis in broiler breeders Avian Pathology: Journal of the W.V.P.A. 37:613–617.
- Aarestrup, F.M., and H. Hasman. 2004. Susceptibility of different bacterial species isolated from food animals to copper sulphate, zinc chloride and antimicrobial substances used for disinfection. Veterinary Microbiology. 100:83–89.
- Johnson, T.J., Y.M. Wannemeuhler, J.A. Scaccianoce, S.J. Johnson, and L.K. Nolan. 2006. Complete DNA sequence, comparative genomics, and prevalence of an IncHI2 plasmid occurring among extraintestinal pathogenic Escherichia coli. Antimicrobial Agents and Chemotherapy. 50:3929–3933.
- Johnson, T.J., D. Jordan, S. Kariyawasam, A.L. Stell, N.P. Bell, Y.M. Wannemuehler, C.F. Alarcon, G. Li, K.A. Tivendale, C.M. Logue, and L.K. Nolan. 2010. Sequence analysis and characterization of a transferable hybrid plasmid encoding multidrug resistance and enabling zoonotic potential for extraintestinal Escherichia coli. Infection and Immunity. 78:1931–1942.
- Johnson, and L.K. Nolan. 2006. Complete DNA sequence, comparative genomics, and prevalence of an IncHI2 plasmid occurring among extraintestinal pathogenic Escherichia coli. Antimicrobial Agents and Chemotherapy. 50:3929–3933
- Skyberg, J.A., K.E. Siek, C. Dotkott, and L.K. Nolan. 2007. Biofilm formation by avian Escherichia coli in relation to media, source and phylogeny. Journal of Applied Microbiology. 102:548–554.
- Mellata, M., J.T. Maddux, T. Nam, N. Thomson, H. Hauser, M.P. Stevens, S. Mukhopadhyay, S. Sarker, A. Crabbe, C.A. Nickerson, J. Santander, and R. Curtiss, 3rd. 2012. New insights into the bacterial fitness-associated mechanisms revealed by the characterization of large plasmids of an avian pathogenic E. coli. PloS One. 7:e29481.
- Musgrove, M.T., D.R. Jones, J.K. Northcutt, N.A. Cox, M.A. Harrison, P.J. Fedorka-Cray, and S.R. Ladely. 2006. Antimicrobial resistance in Salmonella and Escherichia coli isolated from commercial shell eggs. Poultry Science. 85:1665–1669.
- Barrow, P., M. Lovell, and A. Berchieri, Jr. 1998. Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin Diag Lab Immunol. 5:294–298.
- Beery, J.T., M.P. Doyle, and J L. Schoeni. 1985. Colonization of chicken cecae by Escherichia coli associated with hemorrhagic colitis. Applied and Environmental Microbiology. 49:310–315.
- Bayyari, G.R., W.E. Huff, N.C. Rath, J.M. Balog, L.A. Newberry, J.D. Villines, and J.K. Skeeles. 1997. Immune and physiological responses of turkeys with green-liver osteomyelitis complex. Poultry Science. 76:280–288.
- Bazile-Pham-Khac, S., Q.C. Truong, J.P. Lafont, L. Gutmann, X.Y. Zhou, M. Osman, and N.J. Moreau. 1996. Resistance to fluoroquinolones in Escherichia coli isolated from poultry. Antimicrobial Agents and Chemotherapy. 40:1504–1507.
- Bell, C., and A. Kyriakides. 1998. E. coli. A Practical Approach to the Organism and Its Control in Foods. Blackie Academic & Professional, London. 200.
- Berney, M., H.-U. Weilenmann, A. Simonetti, and T. Egli. 2006. Efficacy of solar disinfection of Escherichia coli, Shigella flexneri, Salmonella typhimurium and Vibrio cholerae. Journal of Applied Microbiology. 101:828–836.
- Guttman B, Raya R, Kutter E. Basic phage biology. In: Kutter E, Sulakvelidze A, editors. Bacteriophages biology and applications. Boca Raton: Crc Press; 2005. p. 29–66.
- Hyman P, Abedon ST. Bacteriophage (overview). In: Schaechter M, editor. Desk encyclopedia of microbiology. 2nd ed. Oxford: Elsevier; 2009. p. 166–82.
- https://archive.cnx.org/contents/b4af0cba-3d3c-41c4-aff8-e6235670c885@9/viruses#virus_reproduction.
- Huff WE, Huff GR, Rath NC, Donoghue AM. Critical evaluation of bacteriophage to prevent and treat colibacillosis in poultry. J Ark Acad Sci. 2009;63:93–8.
- Huff, W.E., G.R. Huff, N.C. Rath, J.M. Balog, and A.M. Donoghue. 2004. Therapeutic efficacy of bacteriophage and Baytril (enrofloxacin) individually and in combination to treat colibacillosis in broilers. Poultry Science. 83:1944–1947.
Related topics:
Authors:
Recommend
Comment
Share
14 de marzo de 2023
What is the specific phage against E.coli serotypes ?After phage application for treatment the cure rate % and period of treatment per days?
Recommend
Reply
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.







