Clovibactin, a new peptide antibiotic
Synthesis and Stereochemical Determination of the Peptide Antibiotic Novo29
Author details:
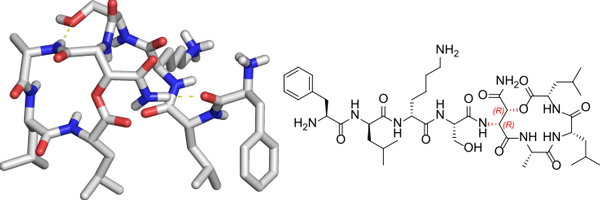
This paper describes the synthesis and stereochemical determination of Novo29 (clovibactin), a new peptide antibiotic that is related to teixobactin and is active against Gram-positive bacteria. Novo29 is an eight-residue depsipeptide that contains the noncanonical amino acid hydroxyasparagine of hitherto undetermined stereochemistry in a macrolactone ring. The amino acid building blocks Fmoc-(2R,3R)-hydroxyasparagine-OH and Fmoc-(2R,3S)-hydroxyasparagine-OH were synthesized from (R,R)- and (S,S)-diethyl tartrate. Novo29 and epi-Novo29 were then prepared by solid-phase peptide synthesis using these building blocks. Correlation with an authentic sample of Novo29 through 1H NMR spectroscopy, LC-MS, and in vitro antibiotic activity established that Novo29 contains (2R,3R)-hydroxyasparagine. X-ray crystallography reveals that epi-Novo29 adopts an amphiphilic conformation, with a hydrophobic surface and a hydrophilic surface. Four sets of epi-Novo29 molecules pack in the crystal lattice to form a hydrophobic core. The macrolactone ring adopts a conformation in which the main-chain amide NH groups converge to create a cavity, which binds ordered water and acetate anion. The amphiphilic conformation of epi-Novo29 is reminiscent of the amphiphilic conformation adopted by the related antibiotic teixobactin and its derivatives, which contains a hydrophobic surface that interacts with the lipids of the bacterial cell membrane and a hydrophilic surface that interacts with the aqueous environment. Molecular modeling suggests that Novo29 can adopt an amphiphilic conformation similar to teixobactin, suggesting that Novo29 may interact with bacteria in a similar fashion to teixobactin.
INTRODUCTION
RESULTS AND DISCUSSION








CONCLUSION
(1) Peoples, A. J.; Hughes, D.; Ling, L. L.; Millett, W.; Nitti, A. G.; Spoering, A.; Steadman, V. A.; Chiva, J. C.; Lazarides, L.; Jones, M. K.; Poullennes, K. G.; Lewis, K.; Epstein, S.″Depsipeptides and uses thereof″. US11,203,616, 2021.
(2) Novobiotic Pharmaceuticals. https://www.novobiotic.com\thescience.
(3) Ling, L. L.; Schneider, T.; Peoples, A. J.; Spoering, A. L.; Engels, I.; Conlon, B. P.; Mueller, A.; Schäberle, T. F.; Hughes, D. E.; Epstein, S.; Jones, M.; Lazarides, L.; Steadman, V. A.; Cohen, D. R.; Felix, C. R.; Fetterman, K. A.; Millett, W. P.; Nitti, A. G.; Zullo, A. M.; Chen, C.; Lewis, K. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455−459.
(4) Ling, L. L.Preclinical Development of Novo29, a New Antibiotic, NIH RePORTER. https://reporter.nih.gov/project-details/ 10111451.
(5) Wirtz, D. A.; Ludwig, K. C.; Arts, M.; Marx, C. E.; Krannich, S.; Barac, P.; Kehraus, S.; Josten, M.; Henrichfreise, B.; Müller, A.; König, G. M.; Peoples, A. J.; Nitti, A. G.; Spoering, A. L.; Ling, L. L.; Lewis, K.; Crüsemann, M.; Schneider, T. Biosynthesis and Mechanism of Action of the Cell Wall Targeting Antibiotic Hypeptin. Angew. Chem., Int. Ed. 2021, 60, 13579−13586.
(6) Yang, H.; Pishenko, A. V.; Li, X.; Nowick, J. S. Design, Synthesis, and Study of Lactam and Ring-Expanded Analogues of Teixobactin. J. Org. Chem. 2020, 85, 1331−1339.
(7) Sieber, P.; Riniker, B. Protection of carboxamide functions by the trityl residue. Application to peptide synthesis. Tetrahedron Lett. 1991, 32, 739−742.
(8) Gao, Y.; Sharpless, K. B. Vicinal diol cyclic sulfates. Like epoxides only more reactive. J. Am. Chem. Soc. 1988, 110, 7538−7539.
(9) He, L.; Byun, H. S.; Bittman, R. Efficient synthesis of chiral α,βepoxyesters via a cyclic sulfate intermediate. Tetrahedron Lett. 1998, 39, 2071−2074.
(10) France, B.; Bruno, V.; Nicolas, I. Synthesis of a protected derivative of (2R,3R)-β-hydroxyaspartic acid suitable for Fmoc-based solid phase synthesis. Tetrahedron Lett. 2013, 54, 158−161.
(11) Guzmán-Martinez, A.; Vannieuwenhze, M. S. An Operationally Simple and Efficient Synthesis of Orthogonally Protected L-threobeta-Hydroxyasparagine. Synlett 2007, 10, 1513−1516.
(12) An 83:12:5 mixture of methyl ester 3, the corresponding diacid precursor, and the corresponding dimethyl ester was used in the next step without further purification.
(13) Liu, L.; Wang, B.; Bi, C.; He, G.; Chen, G. Efficient preparation of β-hydroxy aspartic acid and its derivatives. Chin. Chem. Lett. 2018, 29, 1113−1115.
(14) Chen, K. H.; Le, S. P.; Han, X.; Frias, J. M.; Nowick, J. S. Alanine scan reveals modifiable residues in teixobactin. Chem. Commun. 2017, 53, 11357−11359.
(15) Yang, H.; Chen, K. H.; Nowick, J. S. Elucidation of the Teixobactin Pharmacophore. ACS Chem. Biol. 2016, 11, 1823−1826.
(16) Yang, H.; Du Bois, D. R.; Ziller, J. W.; Nowick, J. S. X-ray crystallographic structure of a teixobactin analogue reveals key interactions of the teixobactin pharmacophore. Chem. Commun. 2017, 53, 2772−2775.
(17) Morris, M. A.; Malek, M.; Hashemian, M. H.; Nguyen, B. T.; Manuse, S.; Lewis, K. L.; Nowick, J. S. A Fluorescent Teixobactin Analogue. ACS Chem. Biol. 2020, 15, 1222−1231.
(18) Yang, H.; Wierzbicki, M.; Du Bois, D. R.; Nowick, J. S. X-ray Crystallographic Structure of a Teixobactin Derivative Reveals Amyloid-like Assembly. J. Am. Chem. Soc. 2018, 140, 14028−14032.
(19) Neises, B.; Steglich, W. Simple Method for the Esterification of Carboxylic Acids. Angew. Chem., Int. Ed. 1978, 17, 522−524.
(20) The macrolactamization reaction proceeds without significant formation of epimers at position 7. The esterification at position 8, however, does result in epimer formation. The epimeric impurity is readily removed during the HPLC purification step.
(21) Independent experiments at NovoBiotic Pharmaceuticals LLC under similar conditions gave MIC values of 16−32 μg/mL for E. coli ATCC 10798.
(22) Shukla, R.; Lavore, F.; Maity, S.; Derks, M. G. N.; Jones, C. R.; Vermeulen, B. J. A.; Melcrová, A.; Morris, M. A.; Becker, L. M.; Wang, X.; Kumar, R.; Medeiros-Silva, J.; van Beekveld, R. A. M.; Bonvin, A. M. J. J.; Lorent, J. H.; Lelli, M.; Nowick, J. S.; MacGillavry, H. D.; Peoples, A. J.; Spoering, A. L.; Ling, L. L.; Hughes, D. E.; Roos, W. H.; Breukink, E.; Lewis, K.; Weingarth, M. Teixobactin kills bacteria by a two-pronged attack on the cell envelope. Nature 2022, 608, 390− 396.
(23) Shukla, R.; Medeiros-Silva, J.; Parmar, A.; Vermeulen, B. J. A.; Das, S.; Paioni, A. L.; Jekhmane, S.; Lorent, J.; Bonvin, A. M. J. J.; Baldus, M.; Lelli, M.; Veldhuizen, E. J. A.; Breukink, E.; Singh, I.; Weingarth, M. Mode of action of teixobactins in cellular membranes. Nat. Commun. 2020, 11, No. 2848.
(24) Ö ster, C.; Walkowiak, G. P.; Hughes, D. E.; Spoering, A. L.; Peoples, A. J.; Catherwood, A. C.; Tod, J. A.; Lloyd, A. J.; Herrmann, T.; Lewis, K.; Dowson, C. G.; Lewandowski, J. R. Structural studies suggest aggregation as one of the modes of action for teixobactin. Chem. Sci. 2018, 9, 8850−8859.
(25) Tayeb-Fligelman, E.; Tabachnikov, O.; Moshe, A.; GoldshmidtTran, O.; Sawaya, M. R.; Coquelle, N.; Colletier, J.; Landau, M. The cytotoxic Staphylococcus aureus PSMα3 reveals a cross-α amyloidlike fibril. Science 2017, 355, 831−833.



















