I. INTRODUCTION
Breeding egg type Japanese quail are responsible for the production of fertile eggs upon which the improved Japanese quail multiplication system for commercial egg production is based. The nutrition of these birds needs to be focused on the production and quality of eggs that are intended for artificial incubation. Maternal nutrition affects egg composition and should provide optimal conditions for embryonic development (Li et al., 2019). This sector has been consolidating as a business activity and, to support this growth, scientific development is needed (Minvielle, 2004), especially in nutrition, which substantially impacts the cost of production.
In this context, lysine (Lys) plays an important role in the protein metabolism of the bird, being considered as a reference amino acid to establish the ideal relation between the essential amino acids, in the concept of ideal protein (Emmert and Baker, 1997). The Lys recommendation values available in the literature have been established for commercial egg producing quail and, to date, no published study has yet demonstrated a difference in Lys requirement between layers and breeders. However, breeding Japanese quail present differences in egg production from quail used for egg production, especially in the sequence of laying and length of pauses, which result in a shorter laying cycle duration. Considering the above, the objective of the study was to estimate the ideal intake of Lys for egg mass production of breeding Japanese quail, using the dilution technique.
II. METHOD
The study was conducted at the Poultry Science Laboratory of the Animal Science Department of the College of Agricultural and Veterinary Sciences of the University Estadual Paulista, Campus of Jaboticabal, São Paulo, Brazil. The Animal Use Ethics Committee approved this study under protocol 012203/17.
Forty-nine breeding Japanese quail females (14 weeks old) in peak health, were housed in galvanized wire cages equipped with trough-type feeders and nipple-type drinkers, with ad libitum water supply. The average temperature during the whole experimental period was 24.5 ºC and the relative humidity was 45% and the light program was 16 hours (h) of light and 8 hours (h) of darkness. A completely randomized design was used, with seven replications and seven treatments of a bird per experimental unit.
Two experimental diets were formulated, one with a high protein content (HPC), with a relative deficiency of 50% Lys, and an excess of 50% concerning the other essential amino acids. The protein-free diet (PFD) was formulated to meet the same nutritional levels as HPC except for crude protein and amino acids. For the formulation of experimental diets, the dilution technique was used (Fischer and Morris, 1970). The intermediate levels were obtained with the dilution between the two diets HPC and PFD, according to the following proportions: HPC: PFD, 100:0; 70:30; 50.1:49.9; 40:60; 30:70; 20.1:79.9; 10:90; thus obtaining Lys concentrations: 14.9, 11.9, 10.4, 7.4, 5.9, 4.4 and 2.9 g / kg, respectively. The trial lasted 22 days, with seven days of adaptation to the facilities and experimental diets and 15 days of data collection. The feed supply was provided according to the maximum expression of the genetic potential, corrected weekly, considering as a basis the metabolic weight of the population and egg production of the respective treatment. The variables collected were: leftover feed, body weight, egg production and egg weight. The variables analysed and the calculated measurements ingestion of Lys and egg output were subjected to orthogonal contrast analysis for the linear and quadratic effect of Lys levels and when the effect was detected (significance level 0.05), the broken line, linear-plateau (BL) models were adjusted and quadratic-plateau (BLq), described, respectively: γ = φ + α [λ - δ] and γ = ξ + β [τ - δ] ², where (λ-δ) and (τ-δ) is 0 for values of δ> λ and δ> τ. γ is the answer; δ is the regressor variable; φ and ξ is the answer corresponding to λ and τ, respectively on the ordinate axis; λ and τ is the point indicating a change in the path of γ corresponding to the abscissa axis; α and β represent the slope, according to Robbins et al. (2006). To determine the ideal Lys intake, the abscissa value corresponding to the first intercept of the quadratic curve was calculated using the linear-plateau model: δ = - (- βλ + sqrt (βφ- βλ)) / β. The parameters were estimated using SAS 9.4 software (SAS Institute Inc., 2014).
III. RESULTS
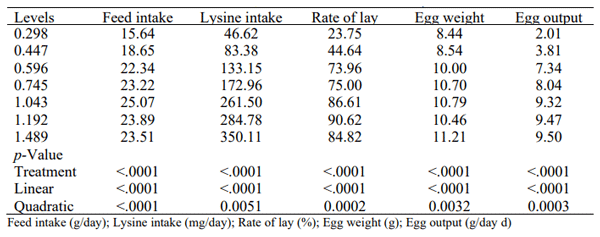
Lys levels affected (p < 0.05) the analyzed variables (Table 1). There were significant linear and quadratic effects of Lys level on all of the analyzed variables. The lower Lys level (0.298%), showed a 60% reduction in feed intake compared to 1.043% of Lys in the diet, which showed higher feed intake. The reduction in Lys intake was 87%, which resulted in a 74% reduction in egg production. Egg production increased up to 1.192% of dietary Lys. The differences between the production variables and egg weight consequently affected egg mass, which was reduced by 79% compared to the lower level of Lys in the diet of 0.298%.
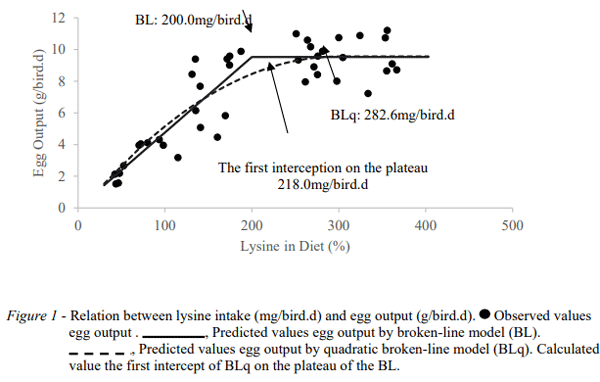
To interpret the linear and quadratic effects of Lys levels, the linear-plateau and quadratic-plateau models were tested (Figure 1). The two equations gave R² values of 0.73 and 0.75 and BIC values of 151 and 223, respectively, indicating that the linear-plateau model provided the best fit for the data. Lys intake estimates were 200 mg/bird d and 283 mg/bird d by the linear-plateau and quadratic-plateau models, respectively. The ideal intake of Lys was calculated at 218 mg/bird d, considering the first interception of the quadratic model in the plateau of the linear model.
Table 1 - Average responses to the dietary levels of lysine
IV. DISCUSSION
The optimal dietary level of Lys of 218 mg/bird d, obtained in this study as the intermediate value between the estimates from the two mathematical models used, can be considered as a robust estimate due to the close fit to the data and the considerable increase in response in egg mass to increase in dietary lysine. (Figure 1).
This study is the first to propose a recommendation for optimal dietary levels of Lys for breeding egg-type Japanese quail. The value of 218 mg/bird d is considerably lower than those documented in the literature, ranging from 292 mg/bird d (Costa et al., 2008) to 321 mg/bird d (Ribeiro et al., 2003). The difference between these studies justifies the conduct of this research and supports the understanding that breeding Japanese quail have differences in the requirement of Lys from commercial egg producing quail. A common practice among poultry nutritionists has been to use recommendations obtained from studies with commercial layer quail for breeders. Based on this study, in addition to offering excess dietary lysine, it is very possible that such diets also contain an excess of other amino acids, due to the fact that lysine is the reference for establishing requirements for the other amino acids. The possible excess of Lys and other amino acids are converted into ammonia, later into uric acid, which has in its synthesis an expensive expenditure of chemical energy (Wu et al., 2013). The greater energy partitioned to eliminate the excess nitrogen can reduce the energy partitioned for egg production.
Increased intake of Lys increased egg weight (Table 1). Increased egg weight for breeding birds invariably affects the hatchability of eggs due to reduced shell thickness (Fouad et al. (2017). A relationship between increased intake of Lys and increased egg weight was observed in duck breeders (Fouad et al., 2017). Although care with egg weight control for breeder eggs is necessary, the values obtained in this research are within the egg weight limit used by the industry, which is between 11 and 12 g.; therefore, the increase described did not provide loss in the hatchability of eggs.
Presented at the 32th Annual Australian Poultry Science Symposium 2021. For information on the next edition, click here.