I. Introduction
Guanidino acetic acid (GAA) is a naturally occurring metabolite which is synthesized in the kidney by L-arginine-glycine amidinotransferase (AGAT) using glycine and arginine (Arg) as substrate (Brosnan et al. 2009). Then, GAA is methylated to creatine in the liver using GAA N-methyltransferase (GAMT). Feeding GAA to humans and animals increases creatine in blood and muscle tissues (Ostoic et al. 2013; DeGroot et al. 2018). High creatine in blood has an inhibitory feedback on AGAT which is known as a rate limiting enzyme in creatine synthesis (Edison et al. 2007, McGuire et al. 1984, Van Pilsem et al. 1971). Thus, GAA is speculated to have Arg sparing effects in broilers. Creatine is an important molecule in energy homeostasis in muscle (Brosnan et al. 2009). In the literature, several groups attempted to research a connection between GAA and efficiency of energy utilization in broilers (Majdeddin et al. 2019; Ale Saheb Fosoul et al. 2018). It is also claimed that GAA can provide 347.5-695 MJ per kg of AMEn (CreAmino, Alzchem, DE; GuanAmino, Evonik, DE) when it is added as a feed additive to a broiler feed. This amount of energy will provide a reduction of 0.21-0.42 MJ/kg of feed by adding 0.06% of GAA in a feed making the diet cheaper. Approximately three quarters of the feed costs are represented by costs for dietary energy. Therefore, an accurate estimation of the energetic value of raw materials is extremely important to reduce the final cost of poultry feed. Prediction of AMEn for different raw materials is affected by the inclusion rate of specific raw materials in the feed (Lopez and Leeson, 2008). Moreover, there is an interaction with other raw materials such as fat and oil. There is also a wide variation in AMEn within and between grain species (Black et al. 2005). For example, the average ileal digestible energy value for different corn samples was 13.42 MJ/kg DM with a standard deviation of 2.04 MJ/kg DM (D'Alfonso, 2005). This high variation can lead to inaccuracy in meeting the energy requirement of birds. Consequently, it creates room for false negative results when a reduction in energy content of feed is under investigation. Herein, the energy sparing effect of GAA is investigated using enough positive and negative controls. Moreover, efficacy of GAA to replace Arg is determined.
II. Method
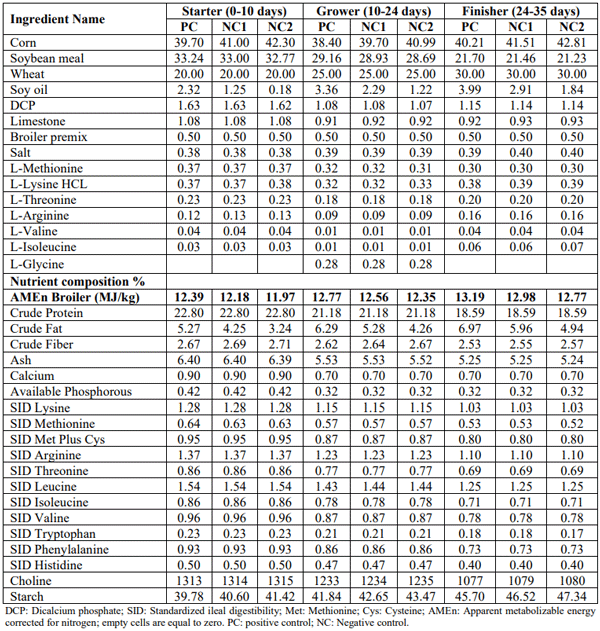
Care and use of animals was in accordance with the principles and guidelines presented in Guide for the Care and Use of Agricultural Animals and Research and Teaching (Federation of Animal Sciences Societies, 2010). Male Ross 308 birds (n=3000) were allocated to 200 pens. Pens were divided between two experiments running in parallel. Experiment 1 consisted of 13 treatment groups. A basal diet deficient in Arg (1.02, 0.88, and 0.75% SID Arg in starter, grower and finisher phases, respectively) was supplemented with 0.06, 0.12, 0.18, 0.30, 0.45, 0.61% of either L-Arg (CJ Europe BestAmino, DE; 98.5% purity) or GAA (Alzchem, DE; 96% purity). All the other nutrients were formulated to meet the Aviagen (2019) recommendations (Table 1). Experiment 2 consisted of 5 treatment groups: T1) A positive control (PC) diet meeting the nutrient specifications according to Aviagen (2019); T2 and T3) Two negative controls (NC1= PC-0.21 MJ/kg AMEn and NC2=PC-0.42 MJ/kg AMEn) to test the sensitivity to lack of energy; T4) 0.06% GAA was added to NC1 diet as a basal; T5) 0.12% GAA was added to NC2 diet as a basal (Table 2).
Table 1 – Basal diets characteristics in the arginine deficient groups (Experiment 1)
Table 2 – Diet characteristics (Experiment 2)
The body weight (BW), DWG, daily feed intake (DFI) and FCR were measured at the end of each growth phase (day 0, 10, 24, and 35). Birds were slaughtered at day 35 (4 birds per pen). Carcass, breast meat and leg weight were measured. Data from experiment 1 were analysed with R (version 3.2.5.). A quadratic polynomial model was used to compare the birds’ response to different doses of Arg and GAA below and above the known Arg requirements (Aviagen, 2019). The dosage of each additive for the maximum performance was estimated and a ratio of the Arg to GAA dose was defined as bio-efficacy in percentage. Data from experiment 2 were analysed using ANOVA (P< 0.05).
III. Results
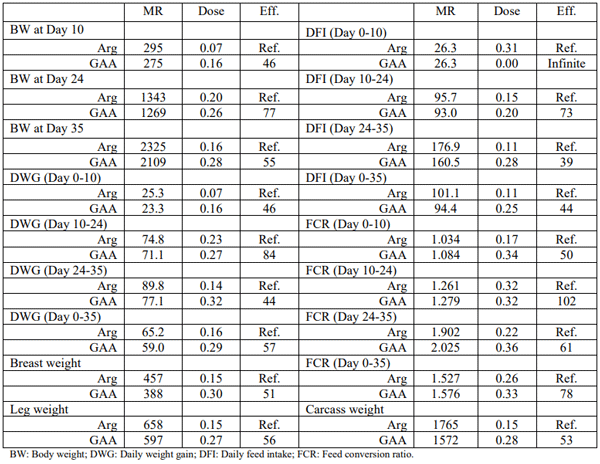
In experiment 1, on average 59.6% of the GAA dose was required to create a comparable performance response with Arg. Approximately 46, 77 and 55% of the GAA dose was needed to achieve the maximum BW when using Arg at the end of starter, grower, and finisher phases, respectively (Table 3). Similarly, to achieve the maximum DWG, approximately 46, 84, 44 and 57% of GAA dose was required during the starter, grower, finisher or during the whole growth period, respectively, to reach a similar response with Arg (Table 3). GAA had a negative impact on BW and DWG at higher doses when added more than 0.18% GAA to an Arg deficient feed. Feed intake was increased in response to Arg or GAA addition. However, GAA had a negative impact on feed intake in an age dependent manner. Efficacy of Arg compared with GAA during the grower, finisher or during the whole growth period was 73, 39, or 44%, respectively (Table 3). During the starter phase, GAA created only a negative impact on feed intake thus Arg efficacy determined to be infinite. Feed conversion ratio was improved by both Arg and GAA. However, GAA had a detrimental impact on FCR at doses higher than 0.18%. Efficacy of GAA vs. Arg was defined equal to 50, 102, 61, and 78% during the starter, grower, finisher and the whole growth period, respectively (Table 3). At day 35, maximum slaughter performances (live weight, carcass weight, breast weight and leg weight) were also achieved with less Arg compared with GAA (56, 53, 51, and 56%, respectively) (Table 3).
Table 3 – Maximum response (MR; gram), the dose required to achieve the MR (%) and the efficacy (Eff.) of GAA (%) to replace Arg (Experiment 1)
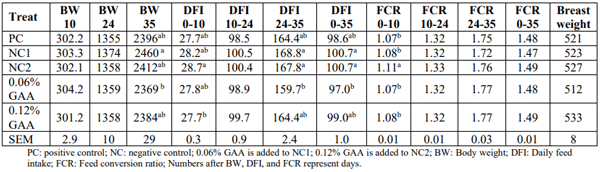
In experiment 2, feed intake was not affected by 0.21 or 0.42 MJ/kg reduction in dietary energy (Table 4). In the starter phase, adding 0.12% GAA to a feed lower in energy by 0.42 MJ/kg caused a reduction in feed intake. In the finisher phase and the entire production period 0-35, a similar reduction in feed intake happened when birds were fed with 0.06% GAA compared to the NC1 diet. FCR was not affected by treatments during the grower, finisher, or the whole growth period. During the starter period, NC2 caused an increase in FCR, but the other groups stayed irresponsive (Table 4). Slaughter data demonstrated that breast meat weight does not respond to any of the treatment groups.
Table 4 – Least square means of body weight (BW; gram), daily feed intake (DFI; gram), feed conversion ratio, and breast weight (gram) in response of treatments (Experiment 2)
IV. Discussion
GAA is a pro-oxidant, a methyl group scavenger, and a precursor of creatine. GAA’s application as a feed additive needs to be carefully monitored because its efficacy is highly depending on availability of methyl donors (EFSA, 2016). Herein, we observed a negative impact of GAA on performance parameters with a dose higher than 0.18% when GAA is added to an Arg deficient diet. The impact of adding comparable amounts of GAA to an Arg adequate diet stays to be elucidated.
Using a semi-purified broiler feed, Dilger et al. (2013) attempted to define the efficiency of GAA. Adding 0.06, 0.12, 0.39, 0.78% GAA to an Arg deficient diet (0.88% Arg) could not match the performance results (212 vs. 145 grams weight gain; 8 to 17 days post hatch) with the deficient diet supplemented with 1% Arg (source of L-Arg was not mentioned: unknown purity). Dilger et al. (2013) in another experiment using a semi-purified diet, compared the efficacy of GAA with Arg using an exponential response model. However, in this experiment in both groups the response was created with graded levels of Arg in two different basal diets: with or without GAA inclusion (0.12% vs. 0%). Dilger et al. (2013) concluded that GAA is an efficacious Arg source under Arg deficient conditions because there was a difference between the two groups when less than 0.4% L-Arg was supplemented to the deficient diet (0.88% SID Arg) and no response was found when more than 0.4% L-Arg was supplemented to the basal diet. According to the current broiler Arg recommendations (1.37% SID Arg in starter broilers; Aviagen, 2019), 0.88% SID Arg is considered a severely deficient diet in Arg. Nevertheless, a quantitative efficacy number was not provided. Herein, we defined the efficacy of GAA and compared it with Arg. On average, GAA could be replaced with 59.6% Arg to achieve a similar maximum performance. During the starter phase, a linear negative impact of GAA on feed intake and a less efficiency of GAA (47.3%) was observed in very young birds.
Broilers response to different levels of energy is highly dependent on the magnitude of energy reduction or addition in the feed. Recent literature has used a stepwise increase or decrease in dietary energy equal to 0.52 to 0.63 MJ/kg feed to test the response to different energy levels (Barekatain et al. 2021; Maharjan et al. 2020). Others have gone one step further and used true metabolized energy (TME) and measured the TME of the applied raw materials to make sure that they will see a response to energy by means of increasing their accuracy (Naranjo, 2018). Lower magnitudes of a change in energy content of feed (AMEn 25-50 kcal/kg of feed) have led to a conclusion that birds do not respond to different energy levels (Dozier and Gehring, 2014). Herein, we also did not observe a response of broilers to a lack of 0.21 or 0.42 MJ AMEn in their feed as compared to the positive control group except for FCR in the starter phase causing a higher FCR, an effect which was not repeated in grower and finisher phase.
GAA is suggested to improve energy homeostasis in muscle tissue because of the data showing extra creatine phosphate and free creatine in muscle tissue (DeGroot et al. 2019). This is often misinterpreted giving GAA an energy matrix value between 83.000 and 166.000 kcal/kg. There is contradiction in the literature regarding the energy saving effect of GAA. On one hand, a negative impact of GAA on growth parameters is observed, especially under deficient or sufficient methionine levels (Majdeddin et al. 2019). On the other hand, a positive impact of GAA is concluded when it is added on top of an energy deficient feed (Ale Saheb Fosoul et al. 2018). Herein, addition of GAA to an energy deficient diet had no positive impact on performance parameters.
In conclusion, the Arg sparing effect of GAA depends on age and on the Arg content of the basal diet. Currently, it is two sized solutions (77% and 149% Arg sparing) for all animal species at different ages. According to our findings, GAA can provide on average 59.6% Arg sparing in broiler chickens. Moreover, GAA is not recommended to be used in young chickens because GAA linearly caused a reduction in their feed intake. In general, a reduction or increase in feed energy levels less than 0.52-0.63 MJ AMEn/kg is a challenge to be measured in broilers. In this study, GAA did not bring any extra energy value into the feed. Thus, it is recommended to use the energy content of GAA as a molecule (10.33 MJ/kg of GAA) instead of an energy sparing value.
Presented at the 33th Annual Australian Poultry Science Symposium 2022. For information on the next edition, click here. 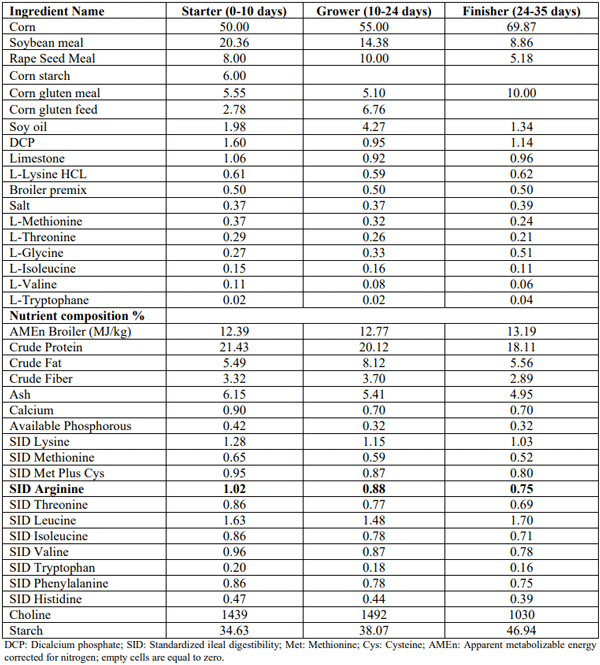










.jpg&w=3840&q=75)