Implications
Nowadays, nearly one out of seven piglets dies before weaning in European commercial herds. Such a high mortality rate is a major source of economic loss and a subject for animal welfare concern. By providing energy and antibodies, colostrum plays an essential role in piglet survival. However, colostrum yield and concentrations of immunoglobulins were shown to be highly variable from sow to sow, and causes for this variability are not fully known yet. A better understanding of factors involved in the variability of colostrum production is a prerequisite before considering developing strategies to improve colostrum production.
Introduction
Pig production is associated with a high rate of piglet mortality between birth and weaning, which reached 14% in France in 2008 (Institut du porc – Gestion Technique des Troupeaux de Truies, IFIP-GTTT, 2009). Most of this mortality is due to a low consumption of colostrum by piglets (Edwards, 2002; Le Dividich et al., 2005). Besides its essential role in providing energy to newborn piglets, colostrum influences the immune competence of piglets. The transfer of immunoglobulins G (IgG) from the colostrum to piglet blood provides passive immunity that contributes to the prevention of disease (Rooke and Bland, 2002). Both colostrum yield and IgG concentrations were shown to be highly variable from sow to sow (Farmer and Quesnel, 2009), even within the breed and among sows reared in similar conditions of housing, feeding and sanitary status (Klobasa and Butler, 1987; Devillers et al., 2007). Unlike milk yield, colostrum yield seems to be only moderately influenced by litter weight and vitality at birth (Devillers et al., 2007). Moreover, it has been suggested that colostrum yield largely depends on the capacity of the sow to produce colostrum. This study was designed to assess the influence of factors related to the litter, the sow and management of colostrum yield and IgG concentrations in a large number of sows. Special focus was given to poorly explored factors such as the process of farrowing and the changes in the integrity of the mammary epithelium, which were shown to play a role in copious colostrum secretion in swine (Foisnet et al., 2010a).
Material and methods
Animals
The experimental animals were reared in compliance with French regulations for the humane care and use of animals in research. The experiment was conducted on 16 Large White (LW) and 56 crossbred Landrace (LR) X Large White (LR X LW) sows of mixed parities. Sows were inseminated by semen from 23 Pietrain boars. During gestation, half the sows were housed individually on a slatted floor and half in groups of six on a concrete floor with straw. The group housing room was equipped with individual feeding stalls. Sows were weighed and backfat thickness was measured ultrasonically (Sonolayer SAL-32B, Toshiba, Tokyo, Japan) at the 10th rib on each side, 65 mm from the midline, just before being transferred to the farrowing room 1 week before the expected date of farrowing. Sows were kept in individual farrowing crates (2 X 2.5 m) on a slatted floor (28% of sows) or on a concrete floor with straw (72% of sows). Sows had free access to water and were fed twice a day on a conventional gestation diet containing 13.2 MJ/kg of digestible energy, 13.3% CP and 0.5% lysine. Daily feed allowance was 2.6 or 2.7 kg for nulliparous sows and between 2.8 and 3.3 kg for primiparous and multiparous sows according to their backfat thickness before insemination. Gestation diet was provided until the second day of lactation. Rectal temperatures were recorded daily at 0900 h during the week of farrowing. The day of farrowing was designated as d0.
Farrowing management and supervision
Parturition was induced by injection of an analogue of prostaglandin F2α (Alfabe´dylR , CEVA Sante´ Animale, Libourne, France, 1 ml intramuscular) on day 113 of gestation, except when parturition had already started on this day or was imminent. Farrowings were attended. Interventions on sows during farrowing were limited as much as possible; oxytocin (BiocytocineR , Laboratoires Biove´, Arques, France) was injected or piglets were manually extracted when latency between two successive births exceeded 60 or 75 min (between the first and second piglet). At birth, each piglet was identified with an ear clip. Each piglet was weighed within 5 min of birth, and then again 24 h after the birth of the first piglet. Stillborn piglets were also weighed at birth. Any abnormal occurrences (i.e. umbilical cord ruptured at delivery, piglet inside placental membranes or difficulty in breathing) that may impair piglet vitality and/or its capacity to reach the teats were recorded at birth. The original litter was kept with the sow during the first 24 h post partum. In addition, piglet death within the first day of life and apparent cause of the death were recorded. Splayleg piglets were also recorded. Castration, iron injection and tattoos were not performed during the first 24 h post partum.
Colostrum sampling
Colostrum samples (30 ml) were obtained immediately after birth of the first piglet and 24 h later (at t0 and t24, respectively). Colostrum was collected from six teats located in the anterior, middle and posterior parts of the udder. At t24, oxytocin (BiocytocineR , Laboratoires Biove´, Arques, France) was injected to induce colostrum ejection. Colostrum samples were immediately filtered through gauze, sampled and stored at -20ºC.
Biological analyses
The concentrations of IgG in the colostrum were analysed in triplicate using a commercial kit (Pig IgG ELISA Quantitation kit, Bethyl Laboratories, Montgomery, USA, ref. E100-104), according to an adaptation by I. Oswald (UR66 Pharmacologie-Toxicologie, INRA, Toulouse, France). The validated procedure was described by Devillers et al. (2004a). Colostrum obtained at t0 and t24 was diluted at 1/420 000 and 1/ 55 000, respectively. The coefficient of variation was 4.7% for a colostrum sample containing 43 mg/ml of IgG.
The contents of Na and K in the colostrum were determined with a Konelab 20i multichannel analyzer with ion selective electrodes after dilution with a diluent for urine (ref. 980303, Thermo Electron Corporation, Cergy Pontoise, France).
Calculations
Individual colostrum intake by newborn pigs between birth and t24 was estimated from piglet weight variation between birth and t24. The equation was proposed by Devillers et al. (2004b): CI = -217.4 + 0.217 X t + 1 861 019 X BW/t + BW0 X (54.8 to 1 861 019/t) X (0.9985 to 3,7 X 10-4 X tfs + 6.1 X 10-7 X tfs2 ; R2 = 0.9), where CI = colostrum intake (g), BW = pig body weight at t24 (kg), BW0 = pig body weight at birth (kg), t = time elapsed from birth to weighing at t24 (min) and tfs = time elapsed from birth to first suckling (min). According to Devillers et al. (2004b), the interval elapsed between birth and first suckling (tfs) can be estimated between 15 and 30 min without major error. In this experiment, an average interval of 30 min was chosen based on experiments previously conducted in the same herd. Piglets that lived ,17 h (22 out of 1027) were excluded from the data set due to inaccurate colostrum intake estimation (Devillers et al., 2004b). The 1005 remaining piglets used to assess colostrum yield were designated as ‘nursed’ piglets. Colostrum production by sows during the 24 h after farrowing started was calculated by summing up colostrum intakes by each piglet of the litter.
The total number of piglets born and the number of piglets born alive, stillborn and nursed were recorded for each litter. The within-litter mean and coefficient of variation of piglet birth weight (CVBW0) were calculated. In addition, the percentage of piglets that had any kind of abnormal occurrence (i.e. umbilical cord ruptured, piglet inside placental membranes, difficulty in breathing or splayleg piglet) at birth or within 24 h from birth was estimated for each litter.
Statistical analyses
All analyses were performed using SAS software (SAS Institute Inc., Cary, NC, USA). Percentages were analysed after arcsin square root transformation. Most analyses were focused on the estimated colostrum yield and IgG concentrations in the colostrum at t0 and t24. The objective was to identify the factors of variation of these parameters among variables related to the sow (i.e. genotype, parity, BW and backfat thickness before farrowing, rectal temperatures on day -1, day 0 and day 1, number of functional teats, gestation length), the litter at birth (i.e. number of total, born alive and stillborn piglets), the nursed litter (i.e. size and weight, average piglet weight at birth, within-litter CVBW0, percentage of piglets with problems) and farrowing process (i.e. induction, duration, average and cumulated interval between births, number of interventions). The type of floor was removed from the analyses because its effect was never significant. Parity was categorised in four classes: 1, 2 to 3, 4 to 5 and 6 or more. Effects of categorised parity, genotype and farrowing induction were analysed using general linear model (proc GLM) procedure, with gestation length and number of nursed piglets included as covariates. Analyses of correlations (proc CORR) and stepwise regression (proc REG) were performed. Correlations were evaluated by using Spearman’s coefficient for the number of stillborn piglets and Pearson’s coefficient otherwise. Analyses of multiple regression included variables that had a normal distribution and thus did not include the number or percentage of stillborn piglets.
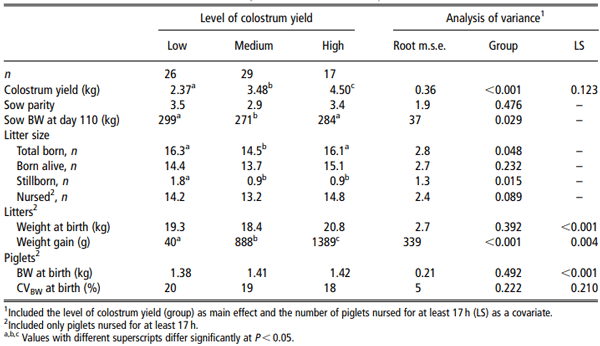
In another approach, sows were categorised into three groups according to their colostrum yield: < 3 kg (Low, n = 26); between 3 and 4 kg (Medium, n = 29) and > 4 kg (High, n = 17). Most litter and sow characteristics within groups were compared by analysis of variance (proc GLM) including colostrum yield as the main factor and the number of nursed piglets as a covariate. When an effect was significant, differences between means were assessed with Scheffe´’s test. Fisher’s exact test was used to compare mortality rate at birth and the percentage of piglets having a problem. Changes in electrolyte concentrations in the colostrum between t0 and t24 were analysed by an analysis for repeated measurements.
The level of significance was P < 0.05. Results are expressed as means ± s.e.m.
Results
Sow and litter characteristics
Sow parity ranked from 1 to 9, with 19% of primiparous and 81% of multiparous sows. Sows weighed 284 ± 5 kg on average before farrowing and had 18.3 ± 0.4 mm of backfat. The number of functional teats varied from 12 to 16 and averaged 14.5 ± 0.1. Gestation length varied from 112 to 115.5 days and averaged 114 ± 0.1 days. Only 12.5% of farrowings were not induced. Most farrowings (n = 56) occurred without human help, while 12 and four farrowings needed one or two interventions, respectively. Farrowing lasted for 275 ± 20 min on average, with a mean interval between births of 19 ± 2 min. Sow rectal temperatures increased daily (P < 0.05) by 0.5°C on average between the day before and the day after farrowing (37.8 ± 0.04, 38.3 ± 0.07 and 38.8 ± 0.09°C on average on day -1, day 0 and day 1, respectively).
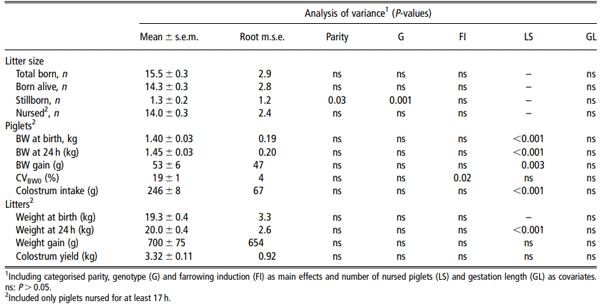
Litter size averaged 14.3 ± 0.3 piglets born alive (Table 1). Stillbirth represented 8% of total piglets. There was an overall effect of categorised parity on stillbirth (P < 0.03; Table 1) but means did not significantly differ between categories. The number of stillborn piglets was 2.1 ± 0.4 and 1.0 ± 0.1 in LW and LR X LW sows, respectively (P z 0.05). The number of piglets nursed by a sow during the first 24 h post partum ranked from 9 to 20 and averaged 14.0 ± 0.3. These litters weighed between 12 and 27 kg. Within-litter CVBW0 averaged 19 ± 1%. It was greater when farrowings were induced (20% v. 16%, P < 0.05).
Piglet weight gain and colostrum intake
The mean piglet weight gain per litter averaged 53 ± 6 g (Table 1) but varied from -320 to +390 g for individual piglets. Similarly, colostrum ingestion was highly variable, from 0 to ±699 g during 24 h. The average ingestion of colostrum within the litter was negatively correlated with the number of nursed piglets (r = -0.45, P < 0.001) and the within-litter CVBW0 (r = -0.30, P = 0.009) and positively correlated with the mean piglet BW at birth (r = 0.35, P = 0.003).
Litter weight gain and estimated colostrum yield
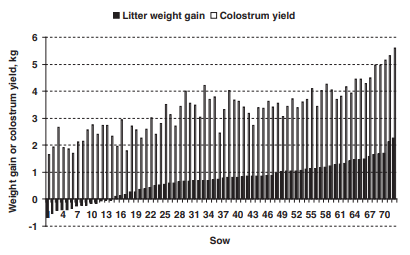
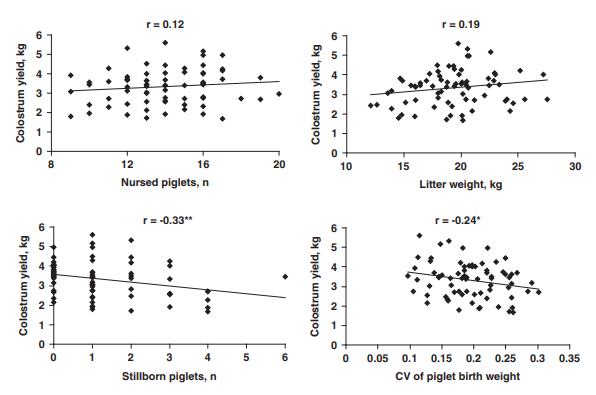
The litter weight gain during the first day post partum averaged 700 ± 75 g (Table 1) but varied from -670 to +2260 g (Figure 1). Twenty percent of the litters lost weight. The estimated colostrum yield in 24 h varied from 1.66 to 5.60 kg and averaged 3.32 ± 0.11 kg (Figure 1). Colostrum yield was not significantly correlated with litter size or weight (P > 0.10; Figure 2). It was negatively correlated with the number of stillborn piglets (r = -0.33, P = 0.005) and CVBW0 (r = -0.24, P = 0.04). Stepwise regression analyses indicated that 6% of variation in colostrum yields was explained by CVBW0 (P = 0.04). No other variables significantly explained part of the variability of colostrum yield.
Concentrations of Na and K in the colostrum
Concentrations of K in the colostrum increased between t0 and t24 (34.6 ± 0.5 v. 38.0 ± 0.5 g/l, P < 0.001), whereas there were decreases in those of Na (36.4 ± 0.9 v. 33.4 ± 0.8 g/l, P < 0.01) as well as the Na/K ratio (1.0 ± 0.1 v. 0.9 ± 0.04, P < 0.01).
Table 1 Descriptive statistics and analysis of variance of litter characteristics (n = 72)
Figure 1 Litter weight gain and estimated colostrum yield during the 24 h post partum (n = 72). Litter weight gain varied from -670 to +2260 g. Estimated colostrum yield in 24 h varied from 1.66 to 5.60 kg and averaged 3.32 ± 0.11 kg.
IgG concentrations in the colostrum
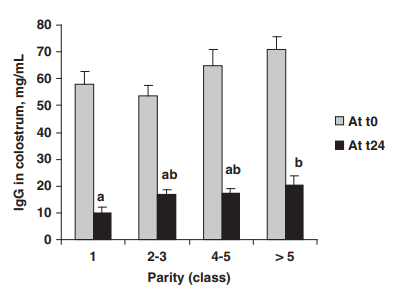
Colostrum concentrations of IgG averaged 62.3 ± 20.1 mg/ml at t0 and 16.8 ± 9.7 mg/ml at t24. They were not significantly (P > 0.1) influenced by sow parity, farrowing induction, litter size and gestation length. Concentrations of IgG were greater (P < 0.01) in LW than in LR x LW sows at t0 (71.7 ± 7.2 and 59.6 ± 2.4 mg/ml, respectively). Within the LR x LW genotype (n = 56), the IgG concentrations were significantly influenced by parity at t24 (P < 0.05) but not at t0 (P = 0.06; Figure 3). At t24, the IgG concentrations were greater in older sows than in primiparous sows (P < 0.05; Figure 3).
In multiple regression analysis, none of the variables significantly explained the variability of IgG concentrations in the colostrum at t0.
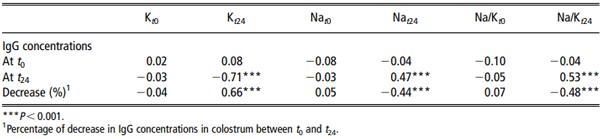
Concentrations of IgG at t24 were not related to concentrations at t0 (P > 0.1). They were negatively correlated with K concentrations in the colostrum at t24 (r = -0.71, P < 0.001) and positively correlated with Na concentrations (r = 0.47, P < 0.001) and the Na/K ratio at t24 (r = 0.53, P < 0.001; Table 2). In multiple regression analysis, 68% of the variability of IgG concentrations at t24 was related to the concentrations of K in the colostrum at t24 (P < 0.001).
Concentrations of IgG decreased on average by 70.9 ± 18.1% between t0 and t24. The amplitude of the decrease was correlated positively with K concentrations (r=0.66, P < 0.001) and negatively with Na concentrations (r = 20.44, P < 0.001) and the Na/K ratio at t24 (r = 20.48, P < 0.001; Table 2).
Comparison of sows and litters according to the level of colostrum yield
Characteristics of nursed litters (size, weight and heterogeneity) and mean piglet birth weight did not significantly differ between sows that produced a Low, Medium or High colostrum yield (Table 3), and nor did the duration of gestation, sow parity, backfat thickness and number of functional teats differ (data not shown). The farrowing duration, average interval between births and number of human interventions did not differ between the three groups of sows (P > 0.1), but the frequency of birth from the second to the 5th piglet tended (P < 0.1) to be lower in the Low than in the Medium and High sows (Figure 4). Sows with a Low colostrum yield were similar to sows with a High colostrum yield with respect to BW and the total number of piglets born; they were heavier and had larger litters than sows with a Medium yield (P < 0.05; Table 3). Sows with a Low colostrum yield had more stillborn piglets (11%) than sows with a Medium (6.2%, P < 0.05) or High colostrum yield (5.7%, P < 0.01). In contrast, they had less (P , 0.05) piglets showing abnormal occurrence (i.e. umbilical cord ruptured, piglet inside placental membranes, difficulty in breathing or splayleg piglet; 2.6%) than sows with a Medium (5.6%) or High (6.6%) colostrum yield. Concentrations of Na and K in the colostrum and the Na/K ratio at t0 and t24 did not differ (P > 0.1) between the three groups of sows (data not shown). Concentrations of IgG at t0 and t24 did not differ between the three groups of sows (P > 0.1).
Figure 2 Relationships between colostrum yield during the 24 h post partum and litter characteristics (number and weight of nursed piglets, number of stillborn piglets and within-litter CV of birth weight). r = coefficient of correlation. *P < 0.05; **P < 0.01.
Figure 3 Effect of parity on concentrations of immunoglobulins G (IgG) in the colostrum collected at the onset of farrowing (t0) and 24 h later (t24) from Landrace x Large White sows (n = 56). a,bWithin the sampling time, means with different superscripts differ (P < 0.05). Mean values were 58 ± 4.9, 53.6 ± 3.7, 65.1 ± 5.8 and 71.1 ± 4.4 mg/ml at t0 and 10.2 ± 2.1, 17.0 ± 1.8, 17.1 ± 1.8 and 20.5 ± 3.4 mg/ml at t24 for parity classes 1, 2 to 3, 4 to 5 and 5 or more, respectively
Discussion
Variability of colostrum yield
This study confirmed that unlike milk production, colostrum production did not increase with litter size. Thus, colostrum intake by piglets was lower when litter size increased, as previously reported (Le Dividich et al., 2005; Devillers et al., 2007). In agreement with previous findings (Devillers et al., 2007), colostrum intake increased with piglet birth weight and decreased when within-litter heterogeneity of birth weight increased. These relationships illustrate the fact that light piglets are often less vigorous and less able to compete with littermates for teat access, and that within-litter heterogeneity most likely exacerbates competition among littermates. Consistently, greater heterogeneity in birth weight within the litter was associated with poorer growth and a higher mortality rate of piglets up to weaning (Milligan et al., 2002; Quiniou et al., 2002).
This study also confirmed the negative relationship between within-litter heterogeneity of birth weight and colostrum yield that was previously reported by Devillers et al. (2007). As mentioned above, a greater heterogeneity within litters may reflect a lower capacity for suckling and therefore less stimulation of teats by piglets. However, in the first hours after farrowing, the colostrum is continuously available (de Passille´ and Rushen, 1989; Fraser and Rushen, 1992) and therefore the influence of suckling stimulus should play only a limited role on milk production during the first day post partum. Besides, the relationship between within-litter heterogeneity of birth weight and colostrum yield was significant but moderate in this study as well as in the previous one (Devillers et al., 2007). Unlike these authors (Devillers et al., 2007), we did not observe significant relationships between mean piglet birth weight or litter weight at birth and colostrum yield.
For the first time, we identified stillbirth as a factor that was negatively related to colostrum yield. The probability of stillbirth was shown to increase with litter size, sow parity and farrowing duration (Fraser et al., 1997; Leenhouwers et al., 1999; Canario et al., 2006; Quesnel et al., 2008).
Table 2 Relationships between immunoglobulins G (IgG) concentrations in colostrum and those of Na and K at the onset of parturition (t0) and 24 h later (t24; Pearson’s coefficients)
Table 3 Characteristics of sows and litters according to the level of colostrum yield
Figure 4 Kinetics of birth of the 14 first piglets born from sows producing a Low, Medium or High yield of colostrum. P-values are 0.02, 0.08 and 0.05 at piglet no. 3, 4 and 5, respectively
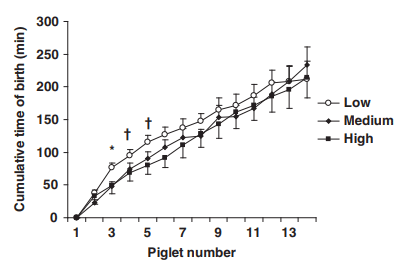
In this study, however, sows that produced a low level of colostrum did not differ from high-producing sows with respect to parity, total number of piglets at birth, number of piglets nursed for 24 h and mean piglet weights at birth, or farrowing duration and mean interval between births. However, the early process of parturition tended to be slower in the low-producing sows than in other sows. The mechanisms underlying the relationship between stillbirth rate and colostrum yield remain to be elucidated. The high rate of stillbirth, related to a slow process of parturition, does not seem to be associated with a lower vitality of piglets at birth, as low-producing sows had less piglets potentially submitted to hypoxia. As most hormones involved in the process of parturition (progesterone, relaxin, oestrogens, glucocorticoids) are also involved in lactogenesis (Farmer and Quesnel, 2009), we may wonder whether alterations of the endocrine state of sows in late pregnancy might have a detrimental effect simultaneously on the process of parturition, stillbirth rate and colostrum production.
Parity and farrowing induction was reported to influence colostrum yield (Devillers et al., 2007). This study did not show significant variations in colostrum yield according to parity. It did not allow the influence of farrowing induction to be assessed, as only 12% of farrowings were not induced. In a recent experiment, however, we concluded that in a herd with an average spontaneous gestation length of 114 days, farrowing can be induced at 113 days of gestation without detrimental effect on colostrum yield (Foisnet et al., 2010b).
The transition from the first step of lactogenesis (before parturition) to copious secretion of colostrum and then of milk is characterised by two main events: a reduction of the permeability of the mammary epithelium due to the development of tight junctions between mammary epithelial cells and an increase in lactose synthesis and excretion into the mammary alveoli (Shennan and Peaker, 2000). A leaky epithelium is incompatible with copious milk secretion in many species (Shennan and Peaker, 2000) and copious production of colostrum in swine (Foisnet et al., 2010a). The increase in the tightness of the mammary epithelium and in lactose secretion induces a decrease in the Na/K ratio in the colostrum, largely due to an increase in K concentrations (Shennan and Peaker, 2000). In this study, the shift in the Na/K ratio occurred, at least partially, within 24 h after the onset of parturition, as previously described (Foisnet et al., 2010c). However, we found no significant relationship between colostrum yield and the Na/K ratio in the colostrum at t24, or the extent of its decrease between t0 and t24. It would be interesting to determine if this shift in the Na/K ratio in lacteal secretion might be related to milk production during early lactation, beyond the first day post partum.
Concentrations of IgG in the colostrum
IgG in the colostrum come exclusively from maternal blood in swine (Bourne and Curtis, 1973). Concentrations in the colostrum were reported to be lower in first to third-parity sows and greater in older sows (Inoue et al., 1980; Klobasa and Butler, 1987). Our findings were consistent with these observations, at least at t24.
Inoue et al. (1980) observed no differences in IgG concentrations between LW and LR x LW sows. The divergence with our findings might be due to the low number of LW sows in this experiment.
The strong correlation between IgG concentrations in the colostrum at t24 and K concentrations or the Na/K ratio in the colostrum at t24 was previously reported (Foisnet et al., 2010a). The uptake of IgG by mammary glands is presumably mediated by an Fc-specific receptor called FcRn in swine (Schnulle and Hurley, 2003) as in ruminants (for a review, see Barrington et al., 2001). In ruminants, prolactin inhibits the expression of the FcRn, thereby inducing the cessation of IgG transfer to the colostrum (Barrington et al., 2001). Simultaneously, prolactin stimulates lactose synthesis and the closure of tight junctions (Shennan and Peaker, 2000). Therefore, the correlations between concentrations of IgG at t24 and those of Na and K are probably not causal relationships but rather events that are part of the same process that initiates copious milk secretion.
The decline of IgG concentrations varied greatly among sows. In 15% of sows, IgG concentrations only decreased by 50% or less in 24 h, which indicates that colostrogenesis may be prolonged beyond 24 h post partum.
Conclusion
This descriptive study confirmed that colostrum yield was independent of litter size but was influenced by within-litter heterogeneity in piglet birth weight. It pointed out the negative link between stillbirth rate and colostrum yield. Our findings also confirmed that parity explains part of the variability in IgG concentrations in the colostrum. However, no other sow-related variables or milk yield were evidenced as a factor influencing IgG concentrations in the colostrum.
Acknowledgements
The author gratefully thanks M. Passet for managing the experimental phase, and for sample and data collection. M. Passet was supported by funds for apprentices provided by INRA. The author also thanks the staff of the experimental herd for animal care and active participation in farrowing supervision and sample collection, C. David for assaying immunoglobulins, Na and K in the colostrums, and A. Foisnet for advising M. Passet.
This article was originally published in Animal (2011), 5:10, pp 1546–1553. doi:10.1017/S175173111100070X. This is an Open Access article under a Creative Commons Attribution License.