A systematic-review on the role of exogenous enzymes on the productive performance at weaning, growing and finishing in pigs
Author details:
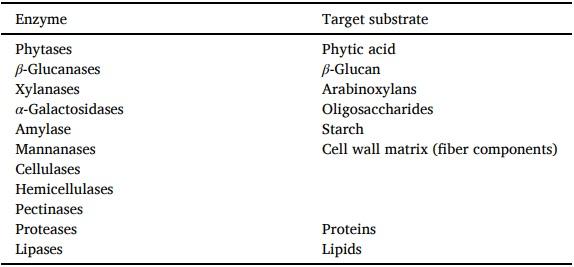
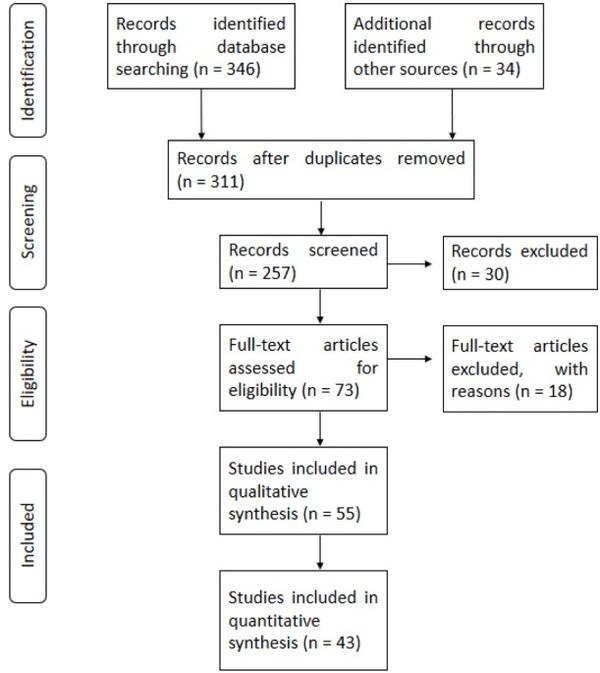
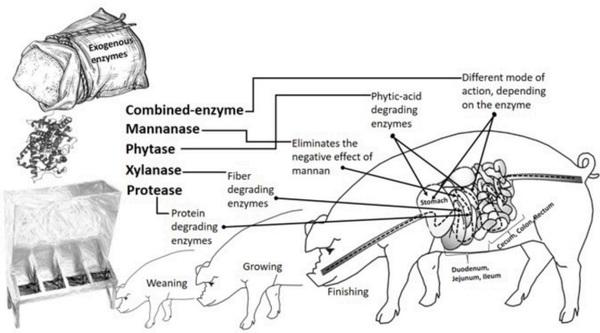

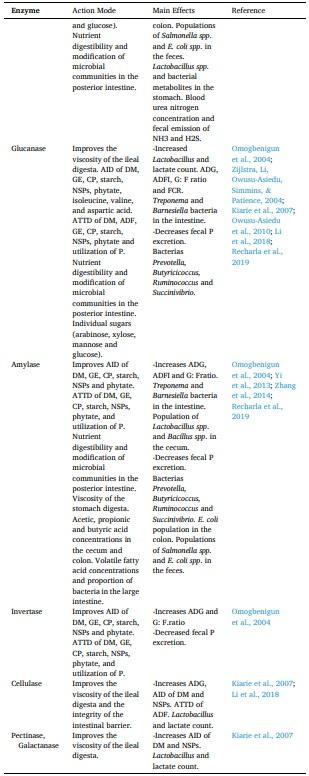
Aarnink, A. J. A., & Verstegen, M. W. A. (2007). Nutrition, key factor to reduce environmental load from pig production. Livestock Science, 109(1–3), 194–203. https://doi.org/10.1016/j.livsci.2007.01.112.
Adeola, O., & Cowieson, A. J. (2011). Board-invited review: Opportunities and challenges in using exogenous enzymes to improve on ruminant animal production.
Journal of Animal Science, 89(10), 3189–3218. https://doi.org/10.2527/jas.2010-
3715.
Agyekum, A. K., Sands, J. S., Regassa, A., Kiarie, E., Weihrauch, D., Kim, W. K., et al. (2015). Effect of supplementing a fibrous diet with a xylanase and β-glucanase blend on growth performance, intestinal glucose uptake, and transport-associated gene expression in growing pigs. Journal of Animal Science, 93(7), 3483–3493. https://doi. org/10.2527/jas.2015-9027.
Ao, X., Meng, Q. W., Yan, L., Kim, H. J., Hong, S. M., Cho, J. H., et al. (2010). Effects of non-starch polysaccharide-degrading enzymes on nutrient digestibility, growth performance and blood profiles of growing pigs fed a diet based on corn and soybean meal. Asian-Australasian Journal of Animal Science, 23(12), 1632–1638. https://doi. org/10.5713/ajas.2010.10123.
Ao, X., Zhou, T. X., Meng, Q. W., Lee, J. H., Jang, H. D., Cho, J. H., et al. (2011). Effects of a carbohydrase cocktail supplementation on the growth performance, nutrient digestibility, blood profiles and meat quality in finishing pigs fed palm kernel meal.
Livestock Science, 137(1–3), 238–243. https://doi.org/10.1016/j.livsci.2010.11.014.
Barrera, M. A., Cervantes, M., Sauer, W. C., Araiza, A. B., & Torrentera, N. (2004). Ileal amino acid digestibility and performance of growing pigs fed wheat-based diets supplemented with xylanase. Journal of Animal Sciences, 82(7), 1997–2003. https:// doi.org/10.2527/2004.8271997x.
Campbell, G. L., & Bedford, M. R. (1992). Enzyme application for monogastric feeds: A review. Canadian Journal of Animal Science, 72, 449–466. https://doi.org/10.4141/ cjas92-058.
Castro, A. M. A., Rosales, G. S., Angeles, M. L., Varela, B. D., Landín, M. G., &
Ibargüengoytia, C. J. A. (2011). Fitasa y enzimas fibrolíticas en dietas para cerdos con diferentes sustratos. Revista Mexicana de Ciencias Pecuarias, 2(2), 117–135.
Cho, J. H., & Kim, I. H. (2013). Effects of beta mannanase and xylanase supplementation in low energy density diets on performances, nutrient digestibility, blood profiles and meat quality in finishing pigs. Asian Journal of animal and Veterinary Advances, 8 (4), 622–630. https://doi.org/10.3923/ajava.2013.622.630.
Cho, J. H., Park, J. H., Lee, D. H., Lee, J. M., Song, T. H., & Kim, I. H. (2017). Effects of xylanase supplementation on growth performance, digestibility, fecal gas emission, and meat quality in growing-finishing pigs. Canadian Journal of Animal Science, 97 (1), 95–100. https://doi.org/10.1139/cjas-2015-0198.
Choct, M. (2015). Feed non-starch polysaccharides for monogastric animals:
Classification and function. Animal Production Science, 55(12), 1360–1366. https:// doi.org/10.1071/AN15276.
Choe, J., Kim, K. S., Kim, H. B., Park, S., Kim, J., & Kim, S (2017). Effect of protease on growth performance and carcass characteristics of growing-finishing pigs. South
African Journal of Animal Science, 47(5), 697–703. https://doi.org/10.4314/sajas. v47i5.13.
Clark, M., & Tilman, D. (2017). Comparative analysis of environmental impacts of agricultural production systems, agricultural input efficiency, and food choice.
Environmental Research Letters, 064016, 12(6), 1–11. https://doi.org/10.1088/1748-
9326/aa6cd5.
Cowieson, A. J., & Bedford, M. R. (2009). The effect of phytase and carbohydrase on ileal amino acid digestibility in monogastric diets: Complementary mode of action?
World´s Poultry Science Journal, 65(4), 609–624. https://doi.org/10.1017/
S0043933909000427.
Da Silva, C. A., Callegari, M. A., Dias, C. P., Bridi, A. M., Pierozan, C. R., Foppa, L., et al. (2019). Increasing doses of phytase from citrobacter braakii in diets with reduced inorganic phosphorus and calcium improve growth performance and lean meat of growing and finishing pigs. PloS one, e0217490, 14(5), 1–13. https://doi.org/
10.1371/journal.pone.0217490.
De Faria, H. G., Thomaz, M. C., Ruiz, U. D. S., Robles-Huaynate, R. A., Watanabe, P. H.,
De Melo, G. M. P., et al. (2015). Effects of phytase on pig diets digestibilities, bone mineral deposition, performance and manure production. Semina: Ciˆencias Agrarias ´
Londrina, 36(6), 4519–4530. https://doi.org/10.5433/1679-
0359.2015v36n6Supl2p4519.
Dersjant-Li, Y., Awati, A., Schulze, H., & Partridge, G. (2014). Phytase in non-ruminant animal nutrition: A critical review on phytase activities in the gastrointestinal tract and influencing factors. Journal of the Science of Food and Agriculture, 95(5),
878–896. https://doi.org/10.1002/jsfa.6998.
Dersjant-Li, Y., Plumstead, P., Awati, A., & Remus, J. (2018). Productive performance of commercial growing and finishing pigs supplemented with a buttiauxella phytase as a total replacement of inorganic phosphate. Animal Nutrition Journal, 4(4), 351–357. https://doi.org/10.1016/j.aninu.2018.02.002.
Dersjant-Li, Y., Schuh, K., Weallean, A. L., Awati, A., & Dusel, G. (2017). Effect of a buttiauxella phytase on production performance in growing/finishing pigs fed a
European-type diet without inclusion of inorganic phosphorus. Journal of Applied
Animal Nutrition, 5(4), 1–7. https://doi.org/10.1017/JAN.2017.3.
Dhawan, S., & Kaur, J. (2007). Microbial mannanases: An overview of production and applications. Critical Reviews in Biotechnology, 27(4), 197–216. https://doi.org/
10.1080/07388550701775919.
European Union Reference Laboratory for Feed Additives (EURL-FA). (2014). Geel,
Belgium. https://ec.europa.eu/food/sites/food/files/safety/docs/oc_eurl_wp_
2015_feed_additives_en.pdf.
EFSA. (2012). Scientific opinion on the safety and efficacy of ronozyme ®HiPhos M/L (6- phytase) as a feed additive for poultry and pigs. EFSA panel on genetically modified organisms (GMO). EFSA Journal, 10(1), 1–12. https://doi.org/10.2903/j. efsa.2012.2527.
He, X., Yu, B., He, J., Huang, Z., Mao, X., Zheng, P., et al. (2020). Effects of xylanase on growth performance, nutrients digestibility and intestinal health in weaned piglets.
Livestock Science, 103940, 233, 1–7. https://doi.org/10.1016/j.livsci.2020.103940.
International Union of Biochemistry and Molecular Biology. (1992). Enzyme nomenclature: Recommendations of the nomenclature committee of international union of biochemistry and molecular biology on the nomenclature and classification of enzymes.
New York: Academic Press. ISBN: 9781483298689.
Jang, J. C., Kim, K. H., Jang, Y. D., & Kim, Y. Y. (2020). Effects of dietary β-mannanase supplementation on growth performance, apparent total tract digestibility, intestinal integrity, and immune responses in weaning pigs. Animals, 10(4), 1–10. https://doi. org/10.3390/ani10040703.
Jha, R., & Berrocoso, J. D. (2015). Review: Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal : An International Journal of
Animal Bioscience, 9(9), 1441–1452, 10.1017%2FS1751731115000919.
Jo, J. K., Ingale, S. L., Kim, J. S., Kim, Y. W., Kim, K. H., Lohakare, J. D., et al. (2012).
Effects of exogenous enzyme supplementation to corn- and soybean meal-based or complex diets on growth performance, nutrient digestibility, and blood metabolites in growing pigs. Journal of Animal Science, 90(9), 3041–3048. https://doi.org/
10.2527/jas.2010-3430.
Jongbloed, A. W., Van Diepen, J. Th. M., Kemme, P. A., & Broz, J. (2004). Efficacy of microbial phytase on mineral digestibility in diets for gestating and lactating sows.
Livestock Production Science, 91(1–2), 143–155. https://doi.org/10.1016/j. livprodsci.2004.07.017.
Juanpere, J., Perez-Vendrell, A. M., Angulo, E., & Brufau, J. (2005). Assessment of potential interactions between phytase and glycosidase enzyme supplementation on nutrient digestibility in broilers. Poulty Science, 84(4), 571–580. https://doi.org/
10.1093/ps/84.4.571.
Kiarie, E., & Nyachoti, C. M. (2009). Alternative feed ingredients in swine diets. In
Proceedings of the 32nd Saskatchewan Pork Industry Symposium, Saskatoon:
Saskatchewan Pork Development Board, 1(1), 29–38.
Kiarie, E., Nyachoti, C. M., Slominski, B. A., & Blank, G. (2007). Growth performance, gastrointestinal microbial activity, and nutrient digestibility in early-weaned pigs fed diets containing flaxseed and carbohydrase enzyme1, 2. Journal of Animal Science, 85 (11), 2982–2993. https://doi.org/10.2527/jas.2006-481.
Kim, B. G., Tian, J. Z., Lim, J. S., Kil, D. Y., Jeon, H. Y., Chung, Y. K., et al. (2004).
Influences of enzyme complex supplementation on growth, ileal and apparent fecal digestibility and morphology of small intestine in pigs. Asian-Australasian Journal of
Animal Sciences, 17(12), 1729–1735. https://doi.org/10.5713/ajas.2004.1729.
Kim, J. C., Sands, J. S., Mullan, B. P., & Pluske, J. R. (2008). Performance and total-tract digestibility responses to exogenous xylanase and phytase in diets for growing pigs.
Animal Feed Science and Technology, 142(1–2), 163–172, 10.1016%2Fj. anifeedsci.2007.07.004.
Kim, J. S., Ingale, S. L., Hosseindoust, A. R., Lee, S. H., Lee, J. H., & Chae, B. J. (2017).
Effects of mannan level and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites of growing pigs. Animal : An
International Journal of Animal Bioscience, 11(2), 202–208. https://doi.org/10.1017/ s1751731116001385.
Kim, J. S., Ingale, S. L., Lee, S. H., Kim, K. H., Kim, J. S., Lee, J. H., et al. (2013). Effects of energy levels of diet and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites in growing pigs. Animal Feed
Science and Technology, 186(1–2), 64–70. https://doi.org/10.1016/j. anifeedsci.2013.08.008.
Lan, R., Li, T., & Kim, I. (2017). Effects of xylanase supplementation on growth performance, nutrient digestibility, blood parameters, fecal microbiota, fecal score and fecal noxious gas emission of weaning pigs fed corn-soybean meal-based diet.
Animal Science Journal, 88(9), 1398–1405. https://doi.org/10.1111/asj.12771.
Lee, S. D., Jung, H. J., Cho, K. H., Park, J. C., Kim, I. C., Seong, P. N., et al. (2011). Effects of corn-dried distiller’s grains with solubles and enzyme premix supplements on growth performance, carcass characteristics and meat quality parameters in finishing pigs. Journal of Animal Science, 82(3), 461–467. https://doi.org/10.1111/j.1740-
0929.2010.00848.x.
Leek, A. B. G., Callan, J. J., Reilly, P., Beattie, V. E., & O’Doherty, J. V. (2007). Apparent component digestibility and manure ammonia emission in finishing pigs fed diets based on barley, maize or wheat prepared without or with exogenous non-starch polysaccharide enzymes. Animal Feed Science and Technology, 135(1–2), 86–99. https://doi.org/10.1016/j.anifeedsci.2006.03.024.
Lei, X. G., Weaver, J. D., Mullaney, E., Ullah, A. H., & Azain, M. J. (2012). Phytase, a new life for an “old” enzyme. Annual Review of Animal Biosciences, 1, 283–309. https:// doi.org/10.1146/annurev-animal-031412-103717.
Lei, X. J., Cheong, J. Y., Park, J. H., & Kim, I. H. (2017). Supplementation of protease, alone and in combination with fructooligosaccharide to low protein diet for finishing pigs. Animal Science Journal, 88(12), 1987–1993. https://doi.org/10.1111/ asj.12849.
Lemme, A., Ravindran, V., & Bryden, W. L. (2004). Ileal digestibility of amino acids in feed ingredients for broilers. World´s Poultry Science Journal, 60(4), 423–437. https:// doi.org/10.1079/WPS200426.
Li, Q., Gabler, N. K., Loving, C. L., Gould, S. A., & Patience, J. F. (2018). A dietary carbohydrase blend improved intestinal barrier function and growth rate in weaned pigs fed higher fiber diets. Journal of Animal Science, 96(12), 5233–5243. https://doi. org/10.1093/jas/sky383.
Li, S., Sauer, W. C., Huang, S. X., & Gabert, V. M. (1996). Effect of β-glucanase supplementation to hulless barley- or wheat-soybean meal diets on the digestibilities of energy, protein,β-glucans, and amino acids in young pigs. Journal of Animal
Science, 74(7), 1649–1656. https://doi.org/10.2527/1996.7471649x.
Lima, M. R., Da Silva, J. H. V., Araujo, J. A., Lima, C. B., & Oliveira, E. R. A. (2007).
Enzimas exogenas ´ na alimentaçao ˜ de aves. Acta Veterinaria Brasilica, 1(4), 99–110. https://doi.org/10.21708/avb.2007.1.4.485.
Lindberg, J. E. (2014). Fiber effects in nutrition and gut health in pigs. Journal of Animal
Science and Biotechnology, 1,5(1), 2–7, 15.10.1186/2049-1891-5-15.
Lu, H., Preynat, A., Legrand-Defretin, V., Geraert, P. A., Adeola, O., & Ajuwon, K. M. (2016). Effects of dietary supplementation of exogenous multi-enzyme mixture containing carbohydrases and phytase on growth performance, energy and nutrient digestibility, and selected mucosal gene expression in the small intestine of weanling pigs fed nutrient deficient diets. Canadian Journal of Animal Science, 96(2), 243–251. https://doi.org/10.1139/cjas-2015-0078.
Lv, J. N., Chen, Y. Q., Guo, X. J., Piao, X. S., Cao, Y. H., & Dong, B. (2013). Effects of supplementation of β-mannanase in corn-soybean meal diets on performance and nutrient digestibility in growing pigs. Asian-Australasian Journal of Animal Sciences,
26(4), 579–587, 10.5713%2Fajas.2012.12612.
Martínez, A. J. A., Figueroa, V. J. L., Cordero, M. J. L., Sanchez, ´ T. E. M. T., &
Martínez, A. M. (2017). Starting pig diets including wheat bran and supplemented with xylanase. Ecosystems and Agricultural Resources Magazine, 4(10), 73–80.
Masey O’Neill, H. V., Smith, J. A., & Bedford, M. R. (2014). Multicarbohydrase enzymes for non-ruminants. Asian-Australasian Journal Animal Sciences, 27(2), 290–301,
10.5713%2Fajas.2013.13261.
Mathlouthi, N., Lalles, J. P., Lepercq, P., Juste, C., & Larbier, M. (2002). Xylanase and beta-glucanase supplementation improve conjugated bile acid fraction in intestinal contents and increase villus size of small intestine wall in broiler chickens fed a ryebased diet. Journal of Animal Science, 80(11), 2773–2779. https://doi.org/
10.2527/2002.80112773x.
Meng, X., & Slominski, B. A. (2005). Nutritive values of corn, soybean meal, canola meal, and peas for broiler chickens as affected by a multicarbohydrase preparation of cell wall degrading enzymes. Poultry Science, 84(8), 1242–1251. https://doi.org/
10.1093/ps/84.8.1242.
Meng, X., Slominski, B. A., Nyachoti, C. M., Campbell, L. D., & Guenter, W. (2005).
Degradation of cell wall polysacxharides by combinations of carbohydrase enzymes and their effect on nutrient utilization and broiler chicken performance. Poultry
Science, 84(1), 37–47. https://doi.org/10.1093/ps/84.1.37.
Nguyen, D. H, et al., Park, J. W., & Kim, I. H.. (2017). Effect of crumbled diet on growth performance, market day age and meat quality of growing-finishing pigs. J. Appl.
Anim. Res, 45, 396–399. https://doi.org/10.1080/09712119.2016.1206904.
Nguyen, D. H., Upadhaya, S. D., Lei, X. J., Yin, J., & Kim, I. H. (2019). Influence of dietary protease supplementation to corn-soybean meal based high and low energy diets on growth performance, nutrient digestibility, blood profiles, and gas emission in growing pigs. Canadian Journal of Animal Science, 99(3), 482–488. https://doi. org/10.1139/cjas-2017-0104.
Nortey, T. N., Patience, J. F., Simmins, P. H., Trottier, N. L., & Zijlstra, R. T. (2007).
Effects of individual or combined xylanase and phytase supplementation on energy, amino acid, and phosphorus digestibility and growth performance of grower swine fed wheat-based diets containing wheat millrun. Journal of Animal Science, 85(6),
1432–1443. https://doi.org/10.2527/jas.2006-613.
Olukosi, O. A., Cowieson, A. J., & Adeola, O. (2007b). Age-related influence of a cocktail of xylanase, amylase, and protease or phytase individually or in combination in broilers. Poultry Science, 86(1), 77–86. https://doi.org/10.1093/ps/86.1.77.
Olukosi, O. A., Sands, J. S., & Adeola, O. (2007a). Supplementation of carbohydrases or phytase individually or in combination to diets for weanling and growing-finishing pigs. Journal of Animal Science, 85(7), 1702–1711. https://doi.org/10.2527/ jas.2006-709.
Omogbenigun, F. O., Nyachoti, C. M., & Slominski, B. A. (2003). The effect of supplementing microbial phytase and organic acids to a corn-soybean based diet fed to early-weaned pigs. Journal of Animal Science, 81(7), 1806–1813. https://doi.org/
10.2527/2003.8171806x.
Omogbenigun, F. O., Nyachoti, C. M., & Slominski, B. A. (2004). Dietary supplementation with multienzyme preparations improves nutrient utilization and growth performance in weaned pigs. Journal of Animal Science, 82(4), 1053–1061. https://doi.org/10.2527/2004.8241053x.
O’Shea, C. J., Mc Alpine, P. O., Solan, P., Curran, T., Varley, P. F., Walsh, A. M., et al. (2014). The effect of protease and xylanase enzymes on growth performance, nutrient digestibility, and manure odour in grower-finisher pigs. Animal Feed Science and Technology, 189, 88–97. https://doi.org/10.1016/j.anifeedsci.2013.11.012.
Owusu-Asiedu, A., Kiarie, E., P´eron, A., Woyengo, T. A., Simmins, P. H., &
Nyachoti, C. M. (2012). Growth performance and nutrient digestibilities in nursery pigs receiving varying doses of xylanase and β-glucanase blend in pelleted wheatand barley-based diets. Journal of Animal Science, 4, 92–94. https://doi.org/
10.2527/jas.51323.
Owusu-Asiedu, A., Simmins, P. H., Brufau, J., Lizardo, R., & P´eron, A. (2010). Effect of xylanase and β-glucanase on growth performance and nutrient digestibility in piglets fed wheat-barley-based diets. Livestock Science, 134(1), 76–78. https://doi.org/
10.1016/j.livsci.2010.06.102.
Park, S., Lee, J. J., Yang, B. M., Cho, J. H., Kim, S., Kang, J., et al. (2020). Dietary protease improves growth performance, nutrient digestibility, and intestinal morphology of weaned pigs. Journal of Animal Science and Technology, 62(1), 21–30. https://doi.org/10.5187/jast.2020.62.1.21.
Parsons, C. M., Castanon, F., & Han, Y. (1997). Protein and amino acid acid digestibility of meat and bone meal. Poultry Science, 76(2), 361–368. https://doi.org/10.1093/ ps/76.2.361.
Passos, A. A., Park, I., Ferket, P., von Heimendahl, E., & Kim, S. W. (2015). Effect of dietary supplementation of xylanase on apparent ileal digestibility of nutrients, viscosity of digesta, and intestinal morphology of growing pigs fed corn and soybean meal based diet. Animal Nutrition, 1(1), 19–23. https://doi.org/10.1016/j. aninu.2015.02.006.
Patience, J.F., .& DeRouchey, J.M. (2010). Feed additives for swine - enzymes and phytase. Animal Science white papers, technical reports, and fact sheets. 13.
Pettey, L. A., Carter, S. D., Senne, B. W., & Shriver, J. A. (2002). Effects of betamannanase addition to corn-soybean meal diets on growth performance, carcass traits, and nutrient digestibility of weanling and growing-finishing pigs. Journal of
Animal Science, 80(4), 1012–1019. https://doi.org/10.2527/2002.8041012x.
Ravindran, V. (2013). Feed enzymes: The science, practice, and metabolic realities.
Journal of Applied Poultry Research, 22(3), 628–636. https://doi.org/10.3382/ japr.2013-00739.
Recharla, N., Kim, D., Ramani, S., Song, M., Park, J., Balasubramanian, B., et al. (2019).
Dietary multi-enzyme complex improves in vitro nutrient digestibility and hind gut microbial fermentation of pigs. PloS one, e02117459, 14(5), 1–19. https://doi.org/
10.1371/journal.pone.0217459.
Rooke, J. A., Slessor, M., Fraser, H., & Thomson, J. R. (1998). Growth performance and gut function of piglets weaned at four weeks of age and fed protease-treated soyabean meal. Animal Feed Science and Technology, 70(3), 175–190. https://doi.org/
10.1016/S0377-8401(97)00083-7.
SAS. (2004). Statistical analysis system, user’s guide. statistical. version (7th ed.). North
Carolina. USA.: SAS. Inst. Inc. Cary.
Sefer, D., Petrujkic, B., Markovic, R., Grdovic, S., Nestorovic, B., Bogosavljevic, V., et al. (2012). Effect of phytase supplementation on growing pigs performance. Acta
Veterinaria Beograd, 62(5–6), 627–639.
Selle, P. H., Cowieson, A. J., & Ravindran, V. (2009). Consequences of calcium interactions with phytase and phytase for poultry and pigs. Livestock Science, 124(1),
126–141.
Selle, P. H., & Ravindran, V. (2008). Phytate-degrading enzymes in pig nutrition.
Livestock Science, 113(2), 99–122. https://doi.org/10.1016/j.livsci.2007.05.014.
Tactacan, G. B., Cho, S. Y., Cho, J. H., & Kim, I. H. (2016). Performance responses, nutrient digestibility, blood characteristics, and measures of gastrointestinal health in weanling pigs fed protease enzyme. Asian Australasian Journal of Animal Science,
29(7), 998–1003. https://dx.doi.org/10.5713%2Fajas.15.0886.
Tsai, T., Dove, C. R., Cline, P. M., Owusu-Asiedu, A., Walsh, M. C., & Azain, M. (2017).
The effect of adding xylanase or β-glucanase to diets with corn distillers dried grains with solubles (CDDGS) on growth performance and nutrient digestibility in nursery pigs. Livestock Science, 197, 4–52. https://doi.org/10.1016/j.livsci.2017.01.008.
Tsai, T. C., Dove, R., Bedford, M. R., & Azain, M. J. (2019). Effect of phytase on phosphorous balance in 20 kg barrows fed low or adequate phosphorous diets.
Animal Nutrition Journal, 6(1), 9–15. https://doi.org/10.1016/j.aninu.2019.11.002.
Upadhaya, S. D., Park, J. W., Lee, J. H., & Kim, I. H. (2016a). Efficacy of β-mannanase supplementation to corn–soya bean meal-based diets on growth performance, nutrient digestibility, blood urea nitrogen, faecal coliform and lactic acid bacteria and faecal noxious gas emission in growing pigs. Archives of Animal Nutrition, 70(1),
33–43. https://doi.org/10.1080/1745039x.2015.1117697.
Upadhaya, S. D., Yun, H. M., & Kim, I. H. (2016b). Influence of low or high density corn and soybean meal-based diets and protease supplementation on growth performance, apparent digestibility, blood characteristics and noxious gas emission of finishing pigs. Animal Feed Science and Technology, 216, 281–287. https://doi.org/
10.1016/j.anifeedsci.2016.04.003.
Veum, T. L., & Odle, J. (2001). Feeding neonatal pigs. In A. J. Lewis, & L. L.. Southern (Eds.), Swine nutrition (pp. 671–690). New York, USA: CRC Press.
Woyengo, T. A., Dupe, V. I. G. E., Akinremi, O. O., & Nyachoti, C. M. (2016).
Performance and nutrient digestibility in growing pigs fed wheat dried distillers’ grain with solubles-containing diets supplemented with phytase and multicarbohydrase. Animal Science Journal, 87(4), 570–577. https://doi.org/10.1111/ asj.12461.
Y´ anez, ˜ J. L., Landero, J. L., Owusu-Asiedu, A., Cervantes, M., & Zijlstra, R. T. (2013).
Growth performance, diet nutrient digestibility, and bone mineralization in weaned pigs fed pelleted diets containing thermostable phytase. Journal of Animal Science, 91 (2), 745–754. https://doi-org.eres.qnl.qa/10.2527/jas.2011-4949.
Yi, J. Q., Piao, X. S., Li, Z. C., Zhang, H. Y., Chen, Y., Li, Q. Y., et al. (2013). The effects of enzyme complex on performance, intestinal health and nutrient digestibility of weaned pigs. Asian Australasian Journal of Animal Science, 26(8), 1181–1188. https://dx.doi.org/10.5713%2Fajas.2013.13129.
Yin, F., Zhang, Z., Huang, J., & Yin, Y. (2010). Digestion rate of dietary starch affects systemic circulation of amino acids in weaned pigs. British Journal of Nutrition, 103 (10), 1404–1412. https://doi.org/10.1017/S0007114509993321.
Yoon, S. Y., Yang, Y. X., Shinde, P. L., Choi, J. Y., Kim, J. S., Kim, Y. W., et al. (2010).
Effects of mannanase and distillers dried grain with solubles on growth performance, nutrient digestibility, and carcass characteristics of grower-finisher pigs. Journal of
Animal Science, 88(1), 181–191. https://doi.org/10.2527/jas.2008-1741.
Zeng, Z., Li, Q., Tian, Q., Zhao, P., Xu, X., Yu, S., et al. (2015). Super high dosing with a novel buttiauxella phytase continuously improves growth performance, nutrient digestibility, and mineral status of weaned pigs. Biological Trace Element Research,
168(1), 103–109. https://doi.org/10.1007/s12011-015-0319-2.
Zeng, Z. K., Piao, X. S., Wang, D., Li, P. F., Xue, L. F., Salmon, L., et al. (2011). Effect of microbial phytase on performance, nutrient absorption and excretion in weaned pigs and apparent ileal nutrient digestibility in growing pigs. Asian-Australasian Journal
Animal Science, 24(8), 1164–1172. https://doi.org/10.5713/ajas.2013.13370.
Zeng, Z. K., Wang, D., Piao, X. S., Li, P. F., Zhang, H. Y., Shi, C. X., et al. (2014). Effects of adding super dose phytase to the phosphorus-deficient diets of young pigs on growth performance, bone quality, minerals and amino acids digestibilities. Asian
Australasian Journal Animal Science, 27(2), 237–246. https://doi.org/10.5713/ ajas.2013.13370.
Zhang, G. G., Yang, Z. B., Wang, Y., Yang, W. R., & Zhou, H. J. (2014). Effects of dietary supplementation of multi-enzyme on growth performance, nutrient digestibility, small intestinal digestive enzyme activities, and large intestinal selected microbiota in weanling pigs. Journal of Animal Science, 92(5), 2063–2069. https://doi.org/
10.2527/jas.2013-6672.
Zhao, J., Zhang, G., Liu, L., Wang, J., & Zhang, S. (2020). Effects of fibre-degrading enzymes in combination with different fibre sources on ileal and total tract nutrient digestibility and fermentation products in pigs. Archives of Animal Nutrition, 74(4),
309–324. https://doi.org/10.1080/1745039X.2020.1766333.
Zijlstra, R. T., Li, S., Owusu-Asiedu, A., Simmins, P. H., & Patience, J. F. (2004). Effect of carbohydrase supplementation of wheat- and canola-meal-based diets on growth performance and nutrient digestibility in group-housed weaned pigs. Canadian
Journal of Animal Science, 84(4), 689–696. https://doi.org/10.4141/A03-127.
Zuo, J., Ling, B., Long, L., Li, T., Lahaye, L., Yang, C., et al. (2015). Effect of dietary supplementation with protease on growth performance, nutrient digestibility, intestinal morphology, digestive enzymes and gene expression of weaned piglets.
Animal Nutrition, 1(4), 276–282. https://doi.org/10.1016/j.aninu.2015.10.003.







.jpg&w=3840&q=75)