Temporal Dynamics of the Gut Bacteriome and Mycobiome in the Weanling Pig
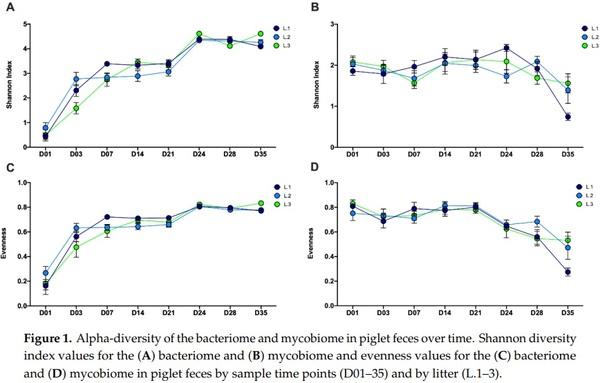
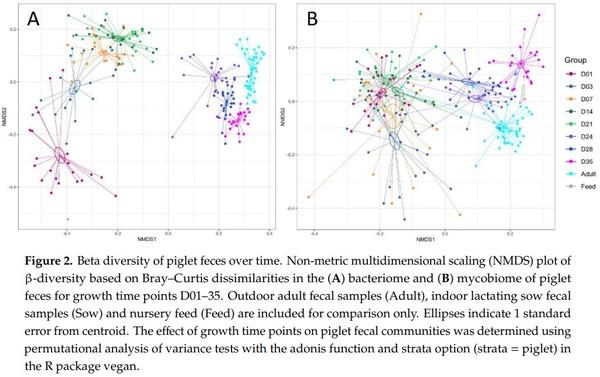
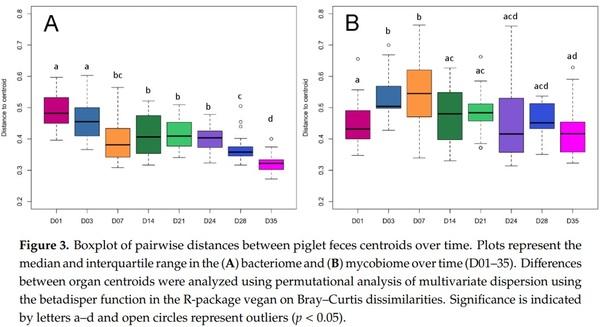
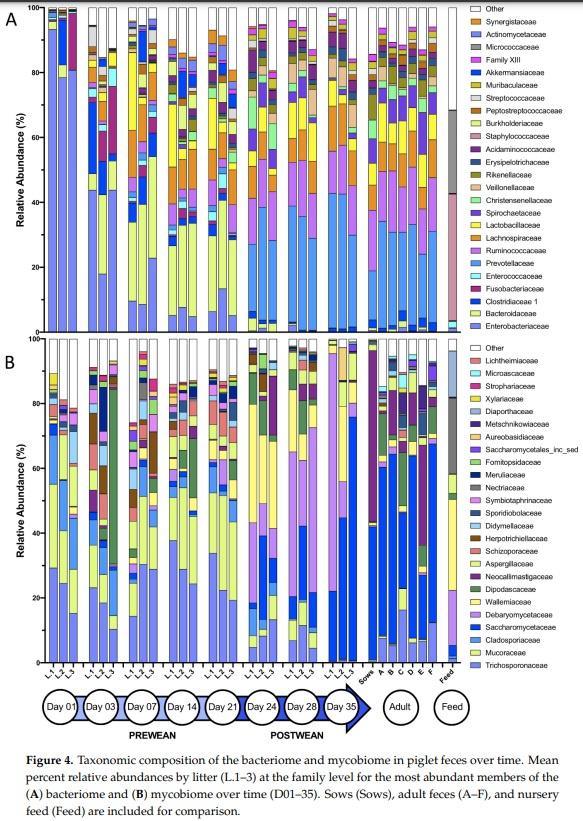
Abstract: Weaning is a period of environmental changes and stress that results in significant alterations to the piglet gut microbiome and is associated with a predisposition to disease, making potential interventions of interest to the swine industry. In other animals, interactions between the bacteriome and mycobiome can result in altered nutrient absorption and susceptibility to disease, but these interactions remain poorly understood in pigs. Recently, we assessed the colonization dynamics of fungi and bacteria in the gastrointestinal tract of piglets at a single time point post-weaning (day 35) and inferred interactions were found between fungal and bacterial members of the porcine gut ecosystem. In this study, we performed a longitudinal assessment of the fecal bacteriome and mycobiome of piglets from birth through the weaning transition. Piglet feces in this study showed a dramatic shift over time in the bacterial and fungal communities, as well as an increase in network connectivity between the two kingdoms. The piglet fecal bacteriome showed a relatively stable and predictable pattern of development from Bacteroidaceae to Prevotellaceae, as seen in other studies, while the mycobiome demonstrated a loss in diversity over time with a post-weaning population dominated by Saccharomycetaceae. The mycobiome demonstrated a more transient community that is likely driven by factors such as diet or environmental exposure rather than an organized pattern of colonization and succession evidenced by fecal sample taxonomic clustering with nursey feed samples post-weaning. Due to the potential tractability of the community, the mycobiome may be a viable candidate for potential microbial interventions that will alter piglet health and growth during the weaning transition.
Keywords: piglet; weaning; microbiome; mycobiome; bacteriome; porcine.
1. Erb Downward, J.R.; Falkowski, N.R.; Mason, K.L.; Muraglia, R.; Huffnagle, G.B. Modulation of post-antibiotic bacterial community reassembly and host response by Candida albicans. Sci. Rep. 2013, 3, 2191. [CrossRef]
[PubMed]
2. Mason, K.L.; Erb Downward, J.R.; Falkowski, N.R.; Young, V.B.; Kao, J.Y.; Huffnagle, G.B. Interplay between the gastric bacterial microbiota and Candida albicans during postantibiotic recolonization and gastritis.
Infect. Immun. 2012, 80, 150–158. [CrossRef] [PubMed]
3. Ott, S.J.; Kuhbacher, T.; Musfeldt, M.; Rosenstiel, P.; Hellmig, S.; Rehman, A.; Drews, O.; Weichert, W.;
Timmis, K.N.; Schreiber, S. Fungi and inflammatory bowel diseases: Alterations of composition and diversity.
Scand. J. Gastroenterol. 2008, 43, 831–841. [CrossRef] [PubMed]
4. Iliev, I.D.; Leonardi, I. Fungal dysbiosis: Immunity and interactions at mucosal barriers. Nat. Rev. Immunol.
2017, 17, 635–646. [CrossRef] [PubMed]
5. Li, X.V.; Leonardi, I.; Iliev, I.D. Gut mycobiota in immunity and inflammatory disease. Immunity 2019, 50,
1365–1379. [CrossRef] [PubMed]
6. Mukherjee, P.K.; Sendid, B.; Hoarau, G.; Colombel, J.F.; Poulain, D.; Ghannoum, M.A. Mycobiota in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 77–87. [CrossRef]
7. Kureljusic, B.; Weissenbacher-Lang, C.; Nedorost, N.; Stixenberger, D.; Weissenbock, H. Association between
Pneumocystis spp. and co-infections with Bordetella bronchiseptica, Mycoplasma hyopneumoniae and
Pasteurella multocida in Austrian pigs with pneumonia. Vet. J. 2016, 207, 177–179. [CrossRef]
8. Weissenbacher-Lang, C.; Kureljusic, B.; Nedorost, N.; Matula, B.; Schiessl,W.; Stixenberger, D.; Weissenbock, H.
Retrospective analysis of bacterial and viral co-infections in pneumocystis spp. positive lung samples of austrian pigs with pneumonia. PLoS ONE 2016, 11, e0158479. [CrossRef]
9. Niederwerder, M.C. Fecal microbiota transplantation as a tool to treat and reduce susceptibility to disease in animals. Vet. Immunol. Immunopathol. 2018, 206, 65–72. [CrossRef]
10. Guevarra, R.B.; Hong, S.H.; Cho, J.H.; Kim, B.R.; Shin, J.; Lee, J.H.; Kang, B.N.; Kim, Y.H.; Wattanaphansak, S.;
Isaacson, R.E.; et al. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J. Anim. Sci. Biotechnol. 2018, 9, 54. [CrossRef]
11. Guevarra, R.B.; Lee, J.H.; Lee, S.H.; Seok, M.J.; Kim, D.W.; Kang, B.N.; Johnson, T.J.; Isaacson, R.E.; Kim, H.B.
Piglet gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechnol. 2019, 10, 1. [CrossRef]
[PubMed]
12. Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol.
2013, 4, 19. [CrossRef] [PubMed]
13. Arfken, A.M.; Frey, J.F.; Ramsay, T.G.; Summers, K.L. Yeasts of burden: Exploring the mycobiome-bacteriome of the piglet GI tract. Front. Microbiol. 2019, 10, 2286. [CrossRef] [PubMed]
14. Urubschurov, V.; Busing, K.; Freyer, G.; Herlemann, D.P.; Souffrant, W.B.; Zeyner, A. New insights into the role of the porcine intestinal yeast, Kazachstania slooffiae, in intestinal environment of weaned piglets.
FEMS Microbiol. Ecol. 2017, 93. [CrossRef] [PubMed]
15. Urubschurov, V.; Busing, K.; Souffrant, W.B.; Schauer, N.; Zeyner, A. Porcine intestinal yeast species,
Kazachstania slooffiae, a new potential protein source with favourable amino acid composition for animals.
J. Anim. Physiol. Anim. Nutr. (Berl.) 2018, 102, e892–e901. [CrossRef]
16. Urubschurov, V.; Janczyk, P.; Pieper, R.; Souffrant, W.B. Biological diversity of yeasts in the gastrointestinal tract of weaned piglets kept under different farm conditions. FEMS Yeast Res. 2008, 8, 1349–1356. [CrossRef]
17. Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [CrossRef]
18. Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [CrossRef]
19. Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.;
Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data.
Nat. Methods 2010, 7, 335–336. [CrossRef]
20. Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.;
Glockner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res.
2014, 42, D643–D648. [CrossRef]
21. Martin, M. Cutadapt removes adaptor sequences from high-throughput sequencing reads. Emb. J. 2011, 17,
10–12. [CrossRef]
22. Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics
2014, 30, 2114–2120. [CrossRef] [PubMed]
23. Koljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.;
Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [CrossRef]
24. Oksanen, J.; Guillaume, B.F.; Michael, F.; Roeland, K.; Pierre, L.; Dan, M.; Peter, R.; Minchin, R.; O’Hara, B.;
Gavin, L.; et al. Vegan: Community Ecology Package. Available online: https://cran.r-project.org (accessed on 15 April 2020).
25. McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [CrossRef] [PubMed]
26. Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Cham,
Switzerland, 2016. [CrossRef]
27. Russel, J.; Thorsen, J.; Brejnrod, A.D.; Bisgaard, H.; Sørensen, S.J.; Burmølle, M. DAtest: A framework for choosing differential abundance or expression method. bioRxiv 2018. [CrossRef]
28. Kurtz, Z.D.; Muller, C.L.; Miraldi, E.R.; Littman, D.R.; Blaser, M.J.; Bonneau, R.A. Sparse and compositionally robust inference of microbial ecological networks. PLoS Comput. Biol. 2015, 11, e1004226. [CrossRef]
29. Csardi, G.; Nepusz, T. The igraph software package for complex network research. InterJ. Complex Syst. 2006,
1695, 1–9.
30. Summers, K.L.; Frey, J.F.; Ramsay, T.G.; Arfken, A.M. The piglet mycobiome during the weaning transition:
A pilot study1. J. Anim. Sci. 2019, 97, 2889–2900. [CrossRef]
31. Frese, S.A.; Parker, K.; Calvert, C.C.; Mills, D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome 2015, 3, 28. [CrossRef]
32. Chen, L.; Xu, Y.; Chen, X.; Fang, C.; Zhao, L.; Chen, F. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front. Microbiol. 2017, 8, 1688. [CrossRef]
33. Vo, N.; Tsai, T.C.; Maxwell, C.; Carbonero, F. Early exposure to agricultural soil accelerates the maturation of the early-life pig gut microbiota. Anaerobe 2017, 45, 31–39. [CrossRef] [PubMed]
34. Konopka, A. What is microbial community ecology? ISME J. 2009, 3, 1223–1230. [CrossRef] [PubMed]
35. Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.;
Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484.
[CrossRef]
36. Raimondi, S.; Amaretti, A.; Gozzoli, C.; Simone, M.; Righini, L.; Candeliere, F.; Brun, P.; Ardizzoni, A.;
Colombari, B.; Paulone, S.; et al. Longitudinal Survey of Fungi in the Human Gut: ITS Profiling, Phenotyping, and Colonization. Front. Microbiol. 2019, 10, 1575. [CrossRef]
37. Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.;
Muzny, D.M.; Gibbs, R.A.; et al. The gut mycobiome of the human microbiome project healthy cohort.
Microbiome 2017, 5, 153. [CrossRef]
38. Kuhbacher, T.; Ott, S.J.; Helwig, U.; Mimura, T.; Rizzello, F.; Kleessen, B.; Gionchetti, P.; Blaut, M.; Campieri, M.;
Folsch, U.R.; et al. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut
2006, 55, 833–841. [CrossRef]
39. Pajarillo, E.A.; Chae, J.P.; Balolong, M.P.; Kim, H.B.; Seo, K.S.; Kang, D.K. Pyrosequencing-based analysis of fecal microbial communities in three purebred pig lines. J. Microbiol. 2014, 52, 646–651. [CrossRef]
40. Alain, B.P.E.; Chae, J.P.; Balolong, M.P.; Bum Kim, H.; Kang, D.K. Assessment of fecal bacterial diversity among healthy piglets during the weaning transition. J. Gen. Appl. Microbiol. 2014, 60, 140–146. [CrossRef]
41. Mach, N.; Berri, M.; Estelle, J.; Levenez, F.; Lemonnier, G.; Denis, C.; Leplat, J.J.; Chevaleyre, C.; Billon, Y.;
Dore, J.; et al. Early-life establishment of the swine gut microbiome and impact on host phenotypes.
Environ. Microbiol. Rep. 2015, 7, 554–569. [CrossRef]
42. Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyotylainen, T.; Hamalainen, A.M.; Peet, A.; Tillmann, V.;
Poho, P.; Mattila, I.; et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 2015, 17, 260–273. [CrossRef]
43. Hallen-Adams, H.E.; Suhr, M.J. Fungi in the healthy human gastrointestinal tract. Virulence 2017, 8, 352–358.
[CrossRef] [PubMed]
44. Ramayo-Caldas, Y.; Prenafeta, F.; Zingaretti, L.M.; Gonzales, O.; Dalmau, A.; Quintanilla, R.; Ballester, M.
Gut eukaryotic communities in pigs: Diversity, composition and host genetics contribution. bioRxiv 2020, 2.
[CrossRef]
45. Colombo, A.L.; Padovan, A.C.; Chaves, G.M. Current knowledge of Trichosporon spp. and Trichosporonosis.
Clin. Microbiol. Rev. 2011, 24, 682–700. [CrossRef] [PubMed]
46. Richardson, M. The ecology of the Zygomycetes and its impact on environmental exposure.
Clin. Microbiol. Infect. 2009, 15 (Suppl. 5), 2–9. [CrossRef]
47. Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P.W. The genus cladosporium. Stud. Mycol. 2012, 72, 1–401.
[CrossRef]
48. Forbes, J.D.; Bernstein, C.N.; Tremlett, H.; Van Domselaar, G.; Knox, N.C. A fungal world: Could the gut mycobiome be involved in neurological disease? Front. Microbiol. 2018, 9, 3249. [CrossRef]
49. Cui, L.; Morris, A.; Ghedin, E. The human mycobiome in health and disease. Genome Med. 2013, 5, 63.
[CrossRef]
50. Hoffmann, C.; Dollive, S.; Grunberg, S.; Chen, J.; Li, H.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Archaea and fungi of the human gut microbiome: Correlations with diet and bacterial residents. PLoS ONE 2013, 8, e66019.
[CrossRef]
51. McCann, A.K.; Barnett, J.A. The utilization of starch by yeasts. Yeast 1986, 2, 109–115. [CrossRef]
52. Kurtzman, C.P. Phylogeny of the ascomycetous yeasts and the renaming of Pichia anomala to
Wickerhamomyces anomalus. Antonie Van Leeuwenhoek 2011, 99, 13–23. [CrossRef]
53. Abe, C.A.; Faria, C.B.; de Castro, F.F.; de Souza, S.R.; dos Santos, F.C.; da Silva, C.N.; Tessmann, D.J.;
Barbosa-Tessmann, I.P. Fungi Isolated from Maize (Zea mays L.) Grains and Production of Associated
Enzyme Activities. Int. J. Mol. Sci. 2015, 16, 15328–15346. [CrossRef] [PubMed]
54. Sam, Q.H.; Chang, M.W.; Chai, L.Y. The fungal mycobiome and its interaction with gut bacteria in the host.
Int. J. Mol. Sci. 2017, 18, 330. [CrossRef] [PubMed]
55. Nogueira, F.; Sharghi, S.; Kuchler, K.; Lion, T. Pathogenetic impact of bacterial-fungal interactions.
Microorganisms 2019, 7, 459. [CrossRef]













