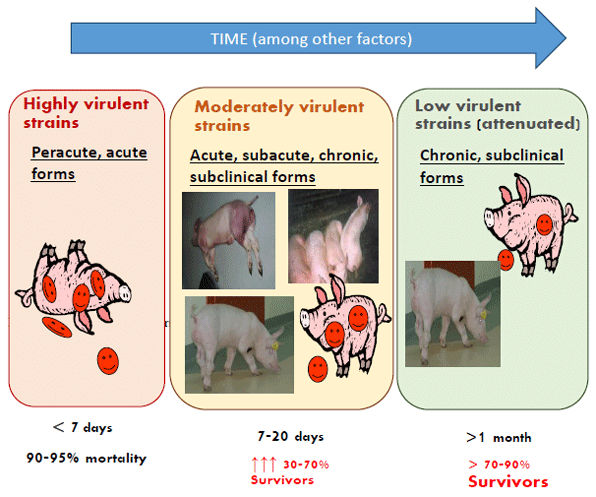
Recognizing African swine fever disease and evolution
References
Achenbach JE, Gallardo C, Nieto-Pelegríın E, Rivera-Arroyo B, Degefa-Negi T, Arias M Sánchez-Vizcaíno JM. Identification of a new genotype of African swine fever virus in domestic pigs from Ethiopia. Transboundary and Emerging Diseases, 2016.
Arias M, Torre A, Dixon L, Gallardo C, Jori F, Laddomada A, Martins C, Parkhouse M, Revilla Y, Rodriguez F, Sánchez-Vizcaíno JM. Approaches and Perspectives for Development of African Swine Fever Virus Vaccines. Vaccines, 5-35, 2017.
Arias M, Jurado C, Gallardo C, Fernández-Pinero J, Sánchez-Vizcaíno JM. Gaps in African swine fever: Analysis and priorities. Transbound Emerg Dis, 65 Suppl 1:235-247, 2018.
Arias M, Sánchez-Vizcaíno J M African swine fever. In A Morilla, KJ Yoon, J Zimmerman (Eds.), Trends in emerging viral infections of swine, 1st ed. pp. 119-124. Ames, IA: Iowa State University Press, 2002.
Atuhaire D K, Afayoa M, Ochwo S, Mwesigwa S, Mwiine F N, Okuni J B, Ojok L. Prevalence of African swine fever virus in apparently healthy domestic pigs in Uganda. BMC Veterinary Research, 9, 263-271, 2013.
Bao J Wang Q Lin P Liu C Li L Wu X Chi T Xu T Ge S Liu Y Li J Wang S Qu H Jin T Wang Z. Genome comparison of African swine fever virus China/2018/AnhuiXCGQ strain and related European p72 Genotype II strains. Transbound Emerg Dis, May;66(3):1167-1176, 2019.
Blome S, Gabriel C, Beer M. Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res, 173 (1), 122-130, 2013
Boinas FS, Wilson AJ, Hutchings GH, Martins C, Dixon LK The persistence of African swine fever virus in fieldinfected Ornithodoros erraticus during the ASF endemic period in Portugal.PLoS One, 6, e20383, 2011 Detray DE African swine fever in warthogs (Phacochoerus aethiopicus). Journal of the American Veterinary Medical Association, 130, 537-540, 1957.
Donaldson TG, Perez de Leon AA, Li AY, Castro-Arellano I, Wozniak E, Boyle WK, Lopez JE Assessment of the geographic distribution of Ornithodoros turicata (Argasidae): Climate variation and host diversity. PLoS Neglected Tropical Diseases, 10,e0004538, 2016.
European Food Safety Authority (EFSA) Scientific opinion on African swine fever (ASF). EFSA Journal, 8, 1-149.2010. http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/1556.pdf. 2010.
Gallardo C, Nieto R, Mur L, Soler A, Pelayo V, Bishop R, Sanchez-Cordon PJ, Martins C, Sanchez-Vizcaino JM, Arias M. African swine fever (ASF) in Africa. In: The role of the African indigenous p¡gs in the transmission of the disease. Brighton, UK: KeyNote, EPIZONE,pp. 12-14, 2012.
Gallardo C, Fernandez-Pinero J, Pelayo V, Gazaev I, Markowska-Daniel I, Pridotkas G, Nieto R, Fernandez-Pacheco P, Bokhan S, Nevolko O, Drozhzhe Z, Perez C, Soler A, Kolvasov D, Arias M. Genetic variation among African swine fever genotype II viruses, eastern and central Europe. Emerg Infect Dis.20(9), 1544-7, 2014.
Gallardo C, Soler A, Nieto R, Sánchez MA, Martins C, Pelayo V, Carrascosa A, Revilla Y, Simón A, Briones V, Sánchez-Vizcaíno JM, Arias M. Experimental Transmission of African Swine Fever (ASF) Low Virulent Isolate NH/P68 by Surviving Pigs. Transbound Emerg Dis. Dec;62(6):612-622, 2015a.
Gallardo MC Reoyo AT, Fernández-Pinero J, Iglesias I, Muñoz MJ, Arias ML. African swine fever: a global view of the current challenge. Porcine Health Manag. Dec 23;1:21, 2015b.
Gallardo C, Nurmoja I, Soler A, Delicado V, Simón A, Martin E, Perez C, Nieto R, Arias M. Evolution in Europe of African swine fever genotype II viruses from highly to moderately virulent. Vet Microbiol. 2018 Jun;219:70-79. doi: 10.1016/j.vetmic.2018.04.001. Epub Apr 7, 2018.
Gallardo C Fernández-Pinero J Arias M. African swine fever (ASF) diagnosis, an essential tool in the epidemiological investigation. 2019a). Virus Res. 2019 Oct 2;271:197676. doi: 10.1016/j.virusres.2019.197676. Epub 2019 Jul 27. Review. PubMed PMID: 31362027, 2019a.
Gallardo C Soler A Rodze I Nieto R Cano-Gómez C Fernandez-Pinero J Arias M. Attenuated and nonhaemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound Emerg Dis, 2019 May;66(3):1399-1404, 2019b.
Ge S Li J Fan X Liu F Li L Wang Q Ren W Bao J Liu C Wang H Liu Y Zhang Y Xu T Wu X Wang Z Molecular Characterization of African Swine Fever Virus, China, 2018. Emerg Infect Dis, Nov;24(11):2131-2133, 2018.
Gómez-Villamandos JC, Hervás J, Méndez A, Carrasco L, Martín de las Mulas J, Villeda CJ, Wilkinson PJ, Sierra MA. Eperimental African swine fever: apoptosis of lymphocytes and virus replication in other cells. Journal of General Virology, 76,pp2399-2405, 1995
Gómez-Villamandos JC, Bautista MJ, Sanchez-Cordon PJ, Carrasco L. Pathology of African swine fever: the role of monocyte-macrophage. Virus Research, 173, pp140-149, 2013.
Groocock CM, Hess WR, Gladney WJ. Experimental transmission of African swine fever virus by Ornithodoros coriaceus, an argasid tick indigenous to United States. American Journal of Veterinary Research, 41, 591-594, 1980.
Haresnape J M, Lungu SAM, Mamu FD. A four-year survey of African swine fever in Malawi.J. Hyg, Camb.95, 309-323,1985.
Haresnape J M; Lungu SAM, Mamu FD. An updated survey of African swine fever in Malawi. Epidem. Inf, 99, 723-732,1987.
Hess WR. African swine fever: a reassessment. Adv Vet Sci Comp Med, 25:39-69. 1981
Hess WR, Endris RG, Haslett TM, Monahan MJ, McCoy JP. Potential arthropod vectors of African swine fever virus in North America and the Caribbean basin. Veterinary Parasitology, 26,145-155.1987
Jori F, Vial L, Penrith M L, Perez-Sanchez R, Etter E, Albina E, Roger F. Review of the sylvatic cycle of African swine fever in sub-Saharan Africa and the Indian Ocean. Virus Research, 173, 212-227. 2013.
Leitão A, Cartaxeiro C, Coelho R, Cruz B, Parkhouse RM, Portugal F, Vigário JD Martins CL. The nonhaemadsorbing African swine fever virus isolate ASFV/NH/P68 provides a model for defining the protective anti-virus immune response. J Gen Virol, Mar;82(Pt 3):513-23, 2001.
Malogolovkin A, Yelsukova A, Gallardo C, Tsybanov S, Kolbasov D. Molecular characterization of African swine fever virus isolates originating from outbreaks in the Russian Federation between 2007 and 2011. Vet Microbiol, 158(3-4), 415-9, 2012.
Mebus CA Dardiri AH Hamdy FM Ferris DH Hess WR Callis JJ. Some characteristics of African swine fever viruses isolated from Brazil and the Dominican Republic. Proc Annu Meet U S Anim Health Assoc, 82:232-6. 1978
Mebus CA, Dardiri AH. Additional characteristics of disease caused by the African swine fever viruses isolated from Brazil and the Dominican Republic. Proceedings of the Annual Meeting of the US Animal Health Association, 227-239. 1979.
Mebus CA, Dardiri AH. Western Hemisphere isolates of African swine fever virus: asymptomatic carriers and resistance to challenge inoculation. Am J.Vet Res, 41:1867-1869, 1980.
Mebus CA, McVicar J W, Dardiri AH. Comparison of the pathology of high and low virulence African swine fever infections. In P.J. Wilkinson (Ed.), African swine fever. Proc. CEC/FAO Research seminar. Sardinia,. 1981.EUR 8466 EN. pp. 183-194. 1983.
Mebus C, Arias M, Pineda J, Tapiador J, House J, Sánchez-Vizcaíno JM. Survival of several porcine viruses in different Spanish dry-cured meat products. Food Chem, 59(4):555-559, 1997
Mellor P S, Wilkinson P. Experimental transmission of African swine fever virus by Ornithodoros savingnyi (Audoin). Research in Veterinary Science, 39, 353-356.1985.
Mur L, Igolkin A, Varentsova A, Pershin A, Remyga S, ShevchenkoI, Sánchez-Vizcaíno J M. Detection of African swine fever antibodies in experimental samples from the Russian Federation:Implications for control. Transboundary and Emerging Diseases, 63, e436-e440,2016
Nurmoja I, Petrov A, Breidenstein C, Zani L, Forth J H, Beer M, Kristian M, Viltrop A, Blome S. Biological characterization of African swine fever virus genotype II strains from north-eastern Estonia in European wild boar. Transbound Emerg Dis, Jan 24, 2017.
Pan I C, Hess WR Virulence in African swine fever: Its measurement and implications. American Journal of Veterinary Research, 45, 361-366, 1984.
Penrith ML Vosloo W. Review of African swine fever: transmission spread and control. J S Afr Vet Assoc.80:58-62,2009
E Okoth, C Gallardo, J M Macharia, A Omore, V Pelayo, D W Bulimo, M Arias, P Kitala, K Baboon, I Lekolol, D Mijele, R P Bishop. Comparison of African swine fever virus prevalence and risk in two contrasting pigfarming systems in South-west and Central Kenya. Prev Vet. Med, 110(2):198-205, 2013.
Quembo CJ, Jori F, Vosloo W, Heath L. Genetic characterization of African swine fever virus isolates from soft ticks at the wildlife/domestic interface inMozambique and identification of a novel genotype. Transbound Emerg Dis,1-12, 2017.
Sánchez-Cordón PJ, Chapman D, Jabbar T, Reis AL, Goatley L, Netherton CL, Taylor G, Montoya M, Dixon L. Different routes and doses influence protection in pigs immunised with the naturally attenuated African swine fever virus isolate OURT88/3. Antiviral Res, Feb;138:1-8, 2017.
Sánchez-BotijaC. African swine fever: New developments. Rev.Sci.Tech, OIE 1:1065-1094,1982
Sánchez-Vizcaíno JM, Laddomada A, Arias M. African swine fever virus. In: Zimmerman, J; Karriker, LA; Ramirez, A.; Schwartz, KJ Stevenson, GW. and Jianquianng Zhang (Eds). Diseases of Swine 11th ed. United States of America: John Wiley and Sons, ISBN:978-1-119-35085-9, chapter 25, pp.443-451, 2019.
Sánchez-Vizcaíno JM; Mur L; Gomez-Villamandos JC, Carrasco L. An update on the epidemiology and pathology of African swine fever. Journal of Comparative Pathology, v.152, p.9-21, 2015.
Uttenthal A Braae UC Ngowi HA Rasmussen TB Nielsen J Johansen MV.ASFV in Tanzania: Asymptomatic pigs harbor virus of molecular similarity to Georgia Vet Microbiol. 2013;165:173-76, 2007.
Arias M Bellini S Westergaard J. Community Veterinary Emergency Team in Lithuania. SCoFCAH (Standing Committee of Food Chain and Animal Health,European Commision. Brussels, EC, August, 14th,2014. http://ec.europa.eu/.
Vlasova N Varentsova A Shevchenko I Zhukov I Remyga S Gavrilova V. Comparative Analysis of Clinical and Biological Characteristics of African Swine Fever Virus Isolates from 2013 Year Russian Federation. British Microbiology Research Journal, 5:203-15, 2015
Zhang H,Cao HW, Wu ZJ, Cui YD. A Review of Molecular Characterization of Classical Swine Fever Virus (CSFV). Israel Journal of Veterinary Medicine, Vol. 66 (3), 2011.













