Public health impact and control options of parasitic zoonoses via pork
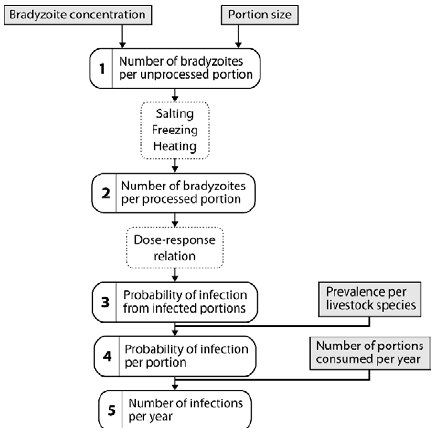
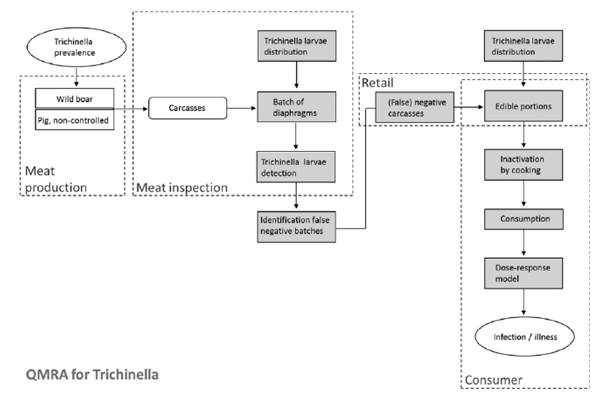
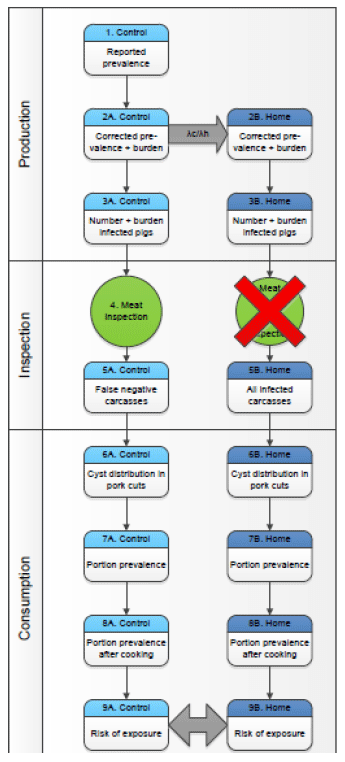
Bouwknegt M, Devleesschauwer B, Graham H, Robertson LJ, van der Giessen JWB, The Euro-Fbp Workshop Participants. Prioritisation of food-borne parasites in Europe, 2016. Eurosurveillance 23(9): 17-00161, 2018.
Deng H, Swart A, Bonacic Marinovic AA, van der Giessen JWB, Opsteegh M. The effect of salting on Toxoplasma gondii viability evaluated and implemented in a quantitative risk assessment of meat-borne human infection. Int J Food Microbiol, 314: 108380, 2019.
Deng H, Swart A, Bonacic AA Marinovic, van der Giessen JWB, Opsteegh M. The effect of salting on Toxoplasma gondii viability evaluated and implemented in a quantitative risk assessment of meat-borne human infection. Int J Food Microbiol, 314: 108380, 2020.
Deng H, Swart A, Wu Y, Li X, Li J, Liu M, Opsteegh M, van der Giessen JWB. Quantitative risk assessment of meat-borne Toxoplasma gondii infection in the mainland of China. Microbial Risk Analysis: 100090, 2019.
Devleesschauwer B, Praet N, Speybroeck N, Torgerson PR, Haagsma JA, De Smet K, Murrell KD, Pozio E, Dorny P. The low global burden of trichinellosis: evidence and implications. Int J Parasitol, 45(2-3): 95-99, 2015.
Franssen F, Takumi K, van der Giessen J, Swart A. Assessing the risk of human trichinellosis from pigs kept under controlled and non-controlled housing in Europe. Food and Waterborne Parasitology, 10: 14-22, 2018.
Franssen F, van Andel E, Swart A, van der Giessen J. Quality Control of Trichinella Testing at the Slaughterhouse Laboratory: Evaluation of the Use of a 400-Micrometer-Mesh-Size Sieve in the Magnetic Stirrer Method. J Food Prot, 79(2): 316-320, 2016.
Guo M, Mishra A, Buchanan RL, Dubey JP, Hill DE, Gamble HR, Jones JL, Du X, Pradhan AK. Development of Dose-Response Models to Predict the Relationship for Human Toxoplasma gondii Infection Associated with Meat Consumption. Risk Anal, 36(5): 926-938, 2016.
Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, Praet N, Bellinger DC, de Silva NR, Gargouri N, Speybroeck N, Cawthorne A, Mathers C, Stein C, FJ Angulo, Devleesschauwer B, World Health Organization Foodborne Disease Burden Epidemiology Reference. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med, 12(12): e1001923, 2015 Meester M, Swart A, Deng H, van Roon A, Trevisan C, Dorny P, Gabriel S, Vieira-Pinto M, Johansen MV, van der Giessen J. A quantitative risk assessment for human Taenia solium exposure from home slaughtered pigs in European countries. Parasit Vectors, 12(1): 82, 2019.
Newell DG, Koopmans M, Verhoef L, E Duizer, Aidara-Kane A, Sprong H, Opsteegh M, Langelaar M, Threfall J, Scheutz F, van der Giessen J, Kruse H. Food-borne diseases - the challenges of 20 years ago still persist while new ones continue to emerge. Int J Food Microbiol, 139 Suppl 1: S3-15, 2010.
Opsteegh M, Prickaerts S, Frankena K, Evers EG. A quantitative microbial risk assessment for meatborne Toxoplasma gondii infection in The Netherlands. International Journal of Food Microbiology, 150(2-3): 103-114, 2011.
Robertson LJ, van der Giessen JWB, Batz MB, Kojima M, Cahill S. Have foodborne parasites finally become a global concern? Trends in Parasitology, 29(3): 101-103, 2013.
Suijkerbuijk AWM, Over EAB, Opsteegh M, Deng H, Gils PF, Bonačić Marinović AA, Lambooij M, Polder JJ, Feenstra TL, Giessen JWB, Wit GA, Mangen M-J J. A social cost-benefit analysis of two One Health interventions to prevent toxoplasmosis. PLOS ONE, 14(5): e0216615, 2019.
Teunis PF, Koningstein M, Takumi K, van der Giessen JW. Human beings are highly susceptible to low doses of Trichinella spp. Epidemiol Infect, 140(2): 210-218, 2012.
Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, Rokni MB, Zhou XN, Fevre, B Sripa EM, Gargouri N, Furst T, Budke CM, Carabin H, Kirk MD, Angulo FJ, Havelaar A, de Silva N. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLoS Med, 12(12): e1001920, 2015.
Trevisan C, Sotiraki S, Laranjo-González M, Dermauw V, Wang Z, Kärssin A, Cvetkovikj A, Winkler AS, Abraham A, Bobić B, Lassen B, Cretu CM, Vasile C, Arvanitis D, Deksne G, Boro I, Kucsera I, Karamon J, Stefanovska J, Koudela B, Pavlova MJ, Varady M, Pavlak M, Šarkūnas M, Kaminski M, Djurković-Djaković O, Jokelainen P, Jan D S, Schmidt V, Dakić Z, Gabriël S, Dorny P, Omeragić J, Alagić D, Devleesschauwer B. Epidemiology of taeniosis/cysticercosis in Europe, a systematic review: eastern Europe. Parasites & vectors, 11(1): 569-569, 2018.
van der Giessen J, Franssen F, Fonville M, Kortbeek T, Beckers P, Tolsma P, Stenvers O, Teunis P, Takumi K. How safe is the meat inspection based on artificial digestion of pooled samples for Trichinella in pork? A scenario from wildlife to a human patient in a non-endemic region of Europe. Vet Parasitol, 194(2-4): 110-112, 2013.
World Health, O, Food and N Agriculture Organization of the United. Multicriteria-based ranking for risk management of food-borne parasites: report of a Joint FAO/WHO expert meeting, 3-7 September 2012, FAO Headquarters, Rome, Italy. Rome, FAO, World Health Organization, 2014.
World Health, O and L F Thomas. Landscape analysis: control of Taenia solium. Geneva, World Health Organization, 2015.







.jpg&w=3840&q=75)