Probiotics Impact on Farmed Animals
Can Probiotics Have a Beneficial Impact on the Health Status of Farmed Animals?

Introduction
Although significant investment is applied to food safety, due to the fact that is of fundamental importance to the economy, food industry and finally to the general consumer, the incidence of foodborne disease is still unacceptably high according to the most recent Community Report based upon the annual reports from the EU Member States and Norway, submitted to the Commission in 2002 (SANCO/56/2003).
The inability to effectively improve this situation in spite of the very significant resources spent is a matter of major concern, calling for novel studies on pathogens to obtain new information and means for their elimination in European food products. The foodborne pathogens dealt with in food and food environmental studies covering the entire food chain, including farmed animals as an integrated food chain, include emerging and reemerging pathogens, like Campylobacter jejuni, Listeria Monocytogenes, verotoxin producing E.coli, Hepatitis E virus and others.
On one hand, breeding animals are exposed to the pathogenic microorganisms and on the other hand, adding antibiotics as feed additives to prevent bacterial diseases and improve animal health and growth are no longer allowed. Moreover, consumers are more and more demanding to eat healthy food and being aware of the animal welfare. The farmers can therefore facing problems concerning the health of farmed animals. However, the role of probiotics in animal health and diseases, is one of the most promising areas of development regarding functional foods. In spite of the fact that most of the research has been focused on the gastrointestinal tract, probiotics can increase the overall animal health performance.
“Probiotics, microorganisms that have a favourable influence on physiologic and pathological processes of the
host by their effect on the intestinal flora, may play a role in improving animal health”. Probiotics may alter farmed animal health by different ways, by 1) improving intestinal barrier and mucosal integrity 2) pathogen exclusion and killing 3) alteration of immune function.
The gut
The epithelium in the intestinal region of a gut consists of heterogeneous population of cells that include enterocytes or absorptive cells, goblet cells that secret mucins, endocrine cells, paneth cells, M cells, tuft and cup cells.
Mucosal surfaces, located inside the body, are a physical barrier between the outside and the sterile interior cavity of the body known as the “systemic” environment. Critical nutrients, oxygen and other molecules are constantly taken up across these mucosal barriers; however, another important function of the mucous is to keep invading pathogens out (Orga et al., 2001). Daily, these mucous membranes are bombarded by outside elements and it is up to the unique immune system of the mucous to determine what is potentially harmful and what is beneficial (Orga et al., 2001; Rosenthal et al., 1997).
The systemic and mucosal immune systems have an arsenal of molecules specifically designed to eliminate invading pathogens. One of the first lines of defense is antibodies, made and secreted by plasma B cells. Antibodies are large protein molecules capable of binding and neutralizing an invading organism. The mucous membranes of the cells produce a special type of antibody called secretory IgA or sIgA.
Another important component of mucosal immunity is the T cell-mediated immune response. T cells that specifically recognize pathogens can help antibodies to clear the infection or directly kill the invader themselves. T cells produced in the mucous are capable of traveling throughout the mucosal tissues through special “homing” receptors on their membranes (McCluskie et al., 1999; Orga et al., 2001; Rosenthal et al., 1997) This means that if an immune response is generated in the gastrointestinal lining, T cells produced there, can travel to other mucosal sites, for example, the lungs or nasal cavity, providing protection over a large surface area (McCluskie et al., 1999; Orga et al., 2001; van Ginkel et al., 2000).
The importance of mucosal immunology lies in the interplay between the mucosal response and the systemic immune response.
Enterocytes
Are the most common epithelial cells in the intestinal epithelium, responsible for majority of the absorption of nutrients and drugs. They are polarised cells with distinct apical and basolateral cytoplasmatic membrane. Enterocytes are separated among themselves by tight junctions and form tight epithelial barrier. Transport of signalling molecules or pathogens themselves across the intestinal epithelial barrier towards underlying mucosal immune system is a complex and dynamic process incuding various functional pathways (transcellular and paracellular transport, transport via carriers and endocytosis) (reviewed by Balimane et al., 2000).
Mucosal lymphatic tissue
The mucosal immune system is the most dispersed, the most diverse and the most complicated lymphocytic system in the body. Like the thymus, it plays a role in generating antigen reactive lymphoid cells which will become specific effectors upon further maturation and on the other hand the mucosal lymphatic tissues are responsible for inducing tolerance to antigens that are commonly experienced in the enteric canal. The mucosal lymphatic tissues are nonencapsulated submucosal lymphoid nodules and diffuse lymphocytic infiltrates in the submucosa of intestinal and respiratory tracts. These organs work collectively with regional lymph nodes and spleen to produce B- and T-effector cells which lodge in lamina propria and in intraepithelial locations wherever there is mucosa (Fig.1).
M-CELLS. The dome epithelium covering each follicle is composed of cuboidal absorptive epithelial cells interrupted by delicate membraneous cells which have luminal microfolds instead of microvillus borders. The “M” cells endocytose and transport various materials without lysomal degradation. Antigen is deposited into lymphocytes, mononuclear phagocytes and dendritic cells immediately beneath M-cells above the B-cell mantle of Peyer’s patch germinal follicles. Minute quantities of intact antigen and products of digestion are transported to the lamina propria and lacteals by ordinary absorptive epithelial cells anywhere in the small bowel. It is important to point out that these products enter interfollicular areas and do not have access to the dome area. Between the dome epithelium and the follicles there is a thin region of reticulum, containing a delicate plexus of blood vessels and plasma cells (Fig.1). The subclass of mucosa-homing T cells, known as intraepithelial lymphocytes, are believed to be involved in protecting mucosal surfaces from injury by infectious pathogens or parasites (London et al., 1994).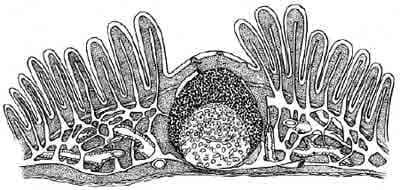

Figure 1. The prototypical Mucosal Lymphatic Tissue is the Peyer’s patch which has a unique dome epithelium that is specialized to sample environmental antigens. Peyer’s patches contain lymphoid compartments that are analogous to the deep cortex and follicles of lymph nodes, but there are no afferent lymphatics and no medullary cords for local accumulation of plasma cells. Each Peyer’s patch contains multiple individual B-cell follicles separated by diffuse lymphoid tissue in interfollicular areas (Parrot, 1976).
FOLLICLES. Peyer’s patch follicles, located beneath the dome epithelium, have a mantle of small B-cells surrounding germinal centers that is thicker facing the dome. The germinal centers contain large and intermediate sized B lymphoblasts, follicular dendritic cells, macrophages and rare T-cells. Regardless how many follicles a patch contain, each is only one follicle thick, providing an intimate association with the overlying epithelium (Fig.1).
INTRAEPITHELIAL LYMPHOCYTES (IEL) are a large heterogeneous population of immune cells in the intestinal epithelium. Two basic characteristics distinguish IEL from peripheral lymphoid cells. First, 80-90X of these cells are CD-8+/CD-4-, of which only half are Thy-l+. In contrast, the predominant phenotype of lamina propria T-cells is CD-4~/CD-8-. Secondly, isolated IEL exhibit in vitro effector functions which identify them as natural killer-like or Cytotoxic cells that are spontaneous or “natural” but without the identifying characteristics of NK cells (Fig.1). In mucosae, cytotoxicity directed toward virus-infected or otherwise parasitized epithelial cells would be prophylactic or protective because the lysed cell would be rapidly eliminated from the body in the mucosal stream. If IgA is also present, it would prevent reinfection of mucosal epithelium.
Host-pathogen interactions
After passing the food chain, food-borne pathogens invade a mammalian body via interactions with the host intestinal tract. Reaching intestinal tract, food-borne pathogens not only interact with the host intestinal barrier but also with commensal microorganisms that colonise intestinal epithelia. Most food-borne pathogens must cross intestinal epithelial barrier to exert their physiopathological effects and to interact with mucosa-associated lymphoid tissue (MALT) (Kerneis et al, 1997). Listeria monocytogenes and Mycobacterium paratuberculosis translocate through M-cells or disrupted tight-epithelia and use host inflammatory response to reorganise enterocytic cytoskeleton and open the paracellular pathway to enhance entrance to the MALT.
Recently, a new mechanism for bacterial uptake in the mucosa has been identified. It has been shown that dendritic cells open the tight-junctions between epithelial cells in order to send dendrites outside the epithelium to directly sample bacteria (reviewed by McCormick, 2003). Increased levels of IFN-gamma in the serum of cattle are used as standardised method for detection of Mycobacterium paratuberculosis in cattle (Hostetter et al., 2002; Kalis et al., 2003). It has also been shown that pathogens may specifically interact with the intestinal epithelium, resulting in trans-cellular signaling cascades to subepithelial neutrophils.
Not only gram positive pathogens can alter the epithelial barrier. It has been shown that enteropathogenic E.coli infection leads to significant decrease in barrier function, loss of microvilli and altered distribution of tight junctional protein ZO-1. Drop in barrier function is accompanied by an increase in flux of paracellular fluid markers (Canil et al., 1993). Thermostable enterotoxin b (STb) is shown to alter intestinal epithelia as analysed by increased Trypan blue uptake (Beausoleil et al., 2002).
Mechanisms of Campylobacter jejuni invasion and pathogenesis are not well understood and thoroughly examined, but seem to be similar to above mentioned pathogens. For the attachment to the host epithelium glycan expression plays a pivotal role (reviewed by Syzmanski et al., 2003). It is believed that C. jejuni translocate the intestinal epithelium through M-cells to reach underlying MALT where it can can survive inside monocytes for up to 7 days (reviewed by Wassenaar and Blaser, 1999; Bereswill and Kist, 2003). The mechanisms of host responses are still not well understood, but it is believed that humoral response occurs in the intestine (reviewed by Wassenaar and Blaser, 1999).
Very few food related studies have been carried out for a human pathogen such as TBEV. Although TBEV is an arbovirus, it is an emerging pathogen, as it was found that approximately 10% of infections were associated with consumption of goat, sheep and cow raw milk and raw milk products (Gritsun et al, 2003; Juceviciene, A. et al., 2002)). TBEV is currently mainly a problem in the Southern and Eastern parts of Europe. Generally the best protection against virus diseases is vaccination (Barrett et al, 2003), however the prophylactic use of probiotics may be a valuable complement to vaccination in endemic TBEV areas, where consumption of raw milk from goats and sheep is common.
With the new lifestyle trends in Europe (more ecological or organic food production, processing and consumption) a potential for much higher incidence of food borne infections by TBEV. Generally the best protection against virus diseases is vaccination (Barrett et al, 2003) however the prophylactic use of probiotics may be a valuable complement to vaccination in endemic TBEV areas, where consumption of raw milk from goats and sheep is common. Probiotics may also be used in prophylactics in western European countries to prevent entry through gastrointestinal tract, especially in those countries (e.g.France) where raw goat milk is used in dairy production.
Hepatitis E virus (HEV) which is a member of the family of caliciviridae is a non-enveloped virus with a positive single-stranded RNA genome. It is the causative agent of hepatitis E in many developing countries in Asia, Africa and Latin America where hepatitis E is an important public health concern. Although HEV is often a self-limiting disease with a relatively low overall death rate (0.5 to 3.0%), the death rate during pregnancy approaches 15 to 25% with possibilities of foetal death, abortion, premature delivery, or death of a live-born baby soon after birth (Smith, 2001). HEV is transmitted primarily by the faecal-oral route and waterborne epidemics are characteristic of hepatitis E.
Recent studies have documented that sporadic acute hepatitis E also occurs in industrialised countries with no history of travel to areas endemic for HEV leading to suggestions that HEV may be endemic at low levels in developed countries. Moreover, cases of acute human hepatitis linked to novel HEV variants have been reported in Europe and Japan (Worm et al., 2002). There are results showing 43,5% sewage samples positive in Barcelona (Spain), 20% in Washington (USA) and 25% in Nancy (France) thus indicating that HEV may be more prevalent that previously considered (Clemente-Casares et al., 2003).
It has been recognised that HEV and HEV related viruses circulate in domestic animals native to industrial countries and should be considered as a potential public health hazard (Worm et al. 2002; Widdowson et al., 2003). This is well documented in swine, but also in chicken . Moreover, it is likely that some foods like shellfish can act as vehicles for transmission of HEV (Smith, 2001). Thus the possibility that farm animal species could represent reservoirs for human contamination through food and meat has to be considered seriously. As an example, a recent report indicate that several patients who contracted sporadic acute or fulminant hepatitis E in Japan had a history of consuming grilled or undercooked pig liver 2-8 weeks before the disease onset (Yazaki et al., 2003).Although HEV is mainly transmitted by the foecal-oral route, the high potential exists for food-borne transmission. Cases of acute hepatites of novel HEV variants have been reported in humans in Europe, Japan thus showing that HEV is not limited and geographically distributed but being endemic (Worm et al., 2002).
There are results showing 43,5% sewage samples positive in Barcelona (Spain), 20% in Washington (USA) and 25% in Nancy (France) that HEV may be more prevalent that previously considered (Clemente-Casares et al., 2003). There has also been an increasing number of reports from Europe and USA of sporadic hepatitis attributable to HEV, leading to suggestions that HEV may be endemic at low levels in developed countries.
Although HEV is often a self-limiting disease, agricultural workers that use untreated wastewater for irrigation are at high risk and mortality of pregnant women (25%) is considerably high (Ceylan et al., 2003; Krawzynski
et al., 2001). It is also of importance that HEV-RNA was found to be present in colostrums of breast-fed mothers that were HEV infected (Chibber et al., 2003). Animal reservoirs like the occurrence of HEV in swine native to industrialised countries should be considered a potential public health hazard and food related studies initiated (Worm et al. 2002; Widdowson et al., 2003).
What do beneficial bacteria (probiotics) do to benefit to the animal health?
Animals form a dynamic, functionally stable and complex ecosystem with microbial community in their gut.
Approximately 400 species of commensal microbial habitants colonise the surface of complex gut epithelium. In fact, the body contains more bacteria than the number of people who have ever lived on the planet. At least the intestine should contain 85% friendly bacteria to prevent the over colonization of disease causing micro-organisms like E. coli and Salmonella. It has been shown that commensal microbes influence the epithelial differentiation and affect the underlying mucous immune system that represents a first line of defence against food borne pathogens by subtile “cross-talk” with the intestinal epithelium (reviewed by Falk et al., 1998).
It was suggested that probiotic (beneficial bacteria) may alter the gut environment, downregulate the mucosal secretory response to pathogens and activate a local immune response (reviewed by Clancy, 2003). Therefore, the commensal microorganisms are essential for the functionality of the host and appreciable in host immune defence. Immune effects of probiotics in man include the stimulation of cell-mediated immune effector functions with enhanced secretion of IFN-gamma by blood cells, enhanced phagocytosis and cell activation (reviewed by Clancy, 2003).
It is important to elucidate the actions of probiotics in their hosts as it was shown that a combination therapy with mixture of probiotics led to inhibition of stimulatory effects of certain species (reviewed by Clancy, 2003). Probiotics can inhibit the growth of harmful bacteria that cause digestive stress, improve digestion of food and absorption of vitamins, stimulate the body’s natural defence mechanism, help to make vitamins needed by the body,…
Why are probiotics supplements necessary for animal health?
Formerly, the intake of probiotic bacteria was high because one of the main methods to preserve the food, was fermentation. Nowadays, the situation is totally different, the intake of probiotic bacteria has dramatically decreased due to the use of freezers, refrigerators, the pasteurisation of food and the use of preservatives like methods of preservation.
The change of life style like, changed diet rich in protein, fat and refined products like sugar and poor fibres, chlorinated drinking water, pollution, stress, increased intake of medicines like antibiotics, birth control pills and many other allopathic drugs cause damage to the intestinal flora and to the tissue in the intestinal wall.
The supplementation of probiotics can increase the nutritional value through better digestibility, increased absorption of minerals and vitamins,…also can have effect in the promotion of intestinal lactose digestion and positive influence on the intestinal microflora (antibiotics or radiation induced colitis). Probiotics prevent the intestinal tract infections (bacteria or virus induced) and help to reduce the diarrhea. Probiotics also play a pivotal role in the regulation of gut motility, improvement of the immune system, reduction of catabolic products eliminated by kidney and liver and improved wellbeing of the animal.
Probiotics and the intestinal barrier
The intestine has a mucosa which works as a selective barrier allowing the passage of useful substances and preventing the penetration of undesirable agents in the bloodstream. Therefore the health of this mucosa is essential to the animal well-being. Probiotic bacteria stimulate the formation of epithelial cells and decrease the inflammation in the intestinal mucosa. The gut barrier function, which protects against the constant exposure to foreign antigens from food and the environment, can be stabilised by probiotic administration.
This is thought to arise from stimulation of production of secretory IgA and mucus and by attenuating pro-inflammatory responses such as IL-8, MCP1, MIP1 and RANTES, pro-inflammatory cytokines (TNF-a, GM-CSF, IL-a and IL-1b) and prostaglandins and leukotrienes induced by pathogens. Probiotics have been administered safely to individuals with immuno-inflammatory disorders such as atopy and Crohn_s disease as well as those with HIV and immunosuppression. Treatment resulted in down-regulation of the overexpressed immuno-inflammatory responses by stimulating regulatory T cells, attenuating pro-inflammatory responses and stabilising the gut mucosal barrier (Rastall et al., 2005).
Probiotics and immunity
Certain investigations have demonstrated that intake of probiotic bacteria increases the production of IgA antibodies and also the activity of macrophages and NK-cells, which leads to an increased killing of bacteria.
Probiotic bacteria also modulate the cytokine activity, which regulate the activity of the immune cells and is a link between the immune system and the nervous system. Administration of probiotic strains causes a range of non-specific and specific host immune responses in diseased and healthy subjects. These include, for example, the enhancement of phagocytic activity of peripheral blood leukocytes and natural killer cell activity.
Additionally, stimulation of both non-specific secretory IgA and specific antibody responses, especially mucosal IgA, to mucosal vaccines such as rotavirus, polio and Salmonella typhi and enteric pathogens such as rotavirus has been seen. Increased cytokine production in vivo (IFN-c, IFN-a, IL-2) and by peripheral blood mononuclear cells ex vivo (IL-1b, TNF-a, IL-6, IL-10, IFNa, IFN-c) have been reported following appropriate probiotic stimulation.
The question is can the reactions be predicted for a given subject, and can they be effectively directed? Few studies have examined the anti-infection effects and host immune responses in the same subjects following
administration of probiotics. Further studies in animals and humans are essential to elucidate the role of probiotics-stimulated immunological mechanisms in protection against enteric pathogens (Rastall et al., 2005).
How can we study the beneficial effects of probiotics?
Germ-free mice are widely used as In vivo experimental system to study the colonisation and defining its underlying mechanisms (reviewed by Falk et al., 1998). Although this system offers rather good model for such studies, it has strong disadvantages: 1) It is not in accordance with the spirit of reduced animal use for research purposes and animal wellfare, 2) It is not suitable for wide laboratory testing, as special facilities and special trained personal is needed, 3) It is expensive and time consuming to obtain results in comparison with good In vitro studies and 4) It is not always a good model to study human pathogen interactions, as some of them can not infect the rodents gut epithelia (e.g. Listeria).
There is also a scientific critisism as animal and human models failed to develop a mechanistic framework. Results obtained in such models were difficult to reproduce, were not clearly linked to clinical outcomes and were outside any physiological framework of host (reviewed by Clancy, 2003). Although the explosive growth in the field genomics and proteomics with technological innovations in recent years will give new data, in vitro models are still essential tools in biological mechanistic studies such as for the accession of cytokine production it is necessary to localise the protein and mRNA. Localisation of protein alone may identify not only producing cells but also target cells and cells which have taken up the protein by endocytosis. mRNA detection only may also
be missleading as some cytokines (like tumor necrosis factor) are regulated posttranscriptionally (Cappelo et al., 1992). Nevertheless, In vitro cell models provided have to satisfy two basic requirements:
1) availability and easy handling for high-throughput testing.
2) retention of tissue characteristics to support interpretation of results for the in vivo situation.
In spite of the fact that primary cells, isolated from human or animal tissue ressemble direct comparability, they survive only few days in cell culture and are difficult to grow (reviewed by Marian, 2002).
So far, human colon tumorigenic cell lines CaCo-2, T84 and HT-29 have been widely used for attachment assays and mechanistic studies, but first they do not derive from the small intestine, second they have tumorigenic phenotype distinguished from the normal gut epithelia and third, they express modified surface glycoconjugates. In addition, in experimental models, they are mostly cultivated as monolayers on plastic surfaces, where the establishment of functional, epithelial character is not defined.
Recently, some laboratories started to use Caco-2 cells grown on the microporous membranes to establish functionality in studies of pathogen translocation (McCormick, 2003). Functional cell models have been successfully used in studies of transepithelial crossing and biological activity of biomolecules mimicing uterine events (Cencic & La Bonnardiere, 2002; Cencic et al. 2002, Cencic et al., 1999) and have been shown they can be used as a good model for studying probiotic-pathogen-gut epithelial interactions (Cencic & Jakobsen, 2002).
Although primary cells, isolated from human or animal tissue, are phenotypically very close to the original tissue, they survive only few days in cell culture and are difficult to grow (reviewed by Marian, 2002).
Pig functional cell model
Pig functional cell model was developed in the framework of PathogenCombat project by A.Cencic and her
group (Gradišnik et al., 2006). To mimic the interactions between model virus, probiotics and piglet intestine, the non-tumorigenic porcine intestinal epithelial cells (IPEC-J2) and alveolar macrophages (3D4/2 and 3D4/21)
were treated in different experimental designs with probiotic and other lactic bacteria and their metabolic products. Vesicular Stomatitis Virus (VSV) was used in the study as a model virus. Cell survival and viral inhibition were determined by antiviral assay and confirmed by immunological methods.
Possible mechanisms of antiviral activity observed: 1) Hindering the adsorption and cell internalisation of the VSV due to the direct trapping of the virus by the bacteria. 2) “Cross-talk” with the cells in establishing the
antiviral protection. 3) Production of metabolites with a direct antiviral effect (Botiæ et al, submitted to IJFM) (Fig.2).
We have shown that preincubation of cell monolayers with probiotic bacteria analysed, reduced viral infectivity up to 60%. First possible mechanism may be interference with virus attachment or entry into the cells, perhaps by steric hindrance (short term pre-incubation).
It was further analysed whether the inhibition of VSV infectivity was due to the adsorption of the virus to probiotic bacteria. We showed that VSV can adsorb with the high affinity to all probiotic strains used in the experiment, that resulted in up to 70% cell survival rate in the IPEC-J2 cell line, whereas lower inhibition of the virus infectivity (~ 50% cell survival) was observed for most of bacteria tested in 3D4/2 cells (Botiæ et al., submitted to the IJFM).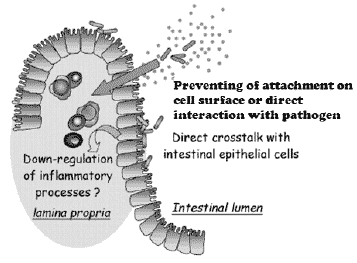
Figure 2. Probiotic bacteria: possible interactions at the intestinal epithelial level. High doses of probiotic bacteria administered by the oral route may allow beneficial effects through the transient colonization of more proximal parts of the gut. As the gut translocation of probiotic microorganisms is probably negligible, the effect of bacterial compounds released in the intestinal lumen and potentially absorbed by the gut epithelium, as well as the direct modulation of the epithelial cell in contact with bacteria have to be considered as possible mechanisms for the development of local or systemic effects of probiotic microorganisms agains pathogenic bacteria and viruses. Furthermore, possible mehanisms that shold not be overmissed is also direct contact of probiotic bacteria with pathogen by trapping them before they reach attachment sit on cells of the host (Heyman et Menard, 2002).
Understanding the role of probiotic bacteria in activation of macrophages and stimulation of proinflammatory cytokine production in early virus infection was studied additionaly in pig alveolar macrophage cell line. It was observed that probiotic bacteria, either from the species Lactobacillus paracasei or Bifidobacteria longum have ability to decrease viral infection by establishing the antiviral state in macrophages, by production of NO and inflammatory cytokines such as interleukin 6 and interferon gamma (Fig.3).
A cell model study used, offers a convenient tool to study virus – host - probiotic bacteria interactions. We showed that probiotic bacteria can exert an antiviral activity against VSV virus. Whereas all lactobacilli of the
paracasei species and B.longum exhibited inhibition of virus infectivity, different mechanisms are involved in antiviral effect that should be elucidated (Botiæ et al., submitted to IJFM). Further studies in functional cell models may help to dissect the detail mechanisms involved in the antiviral potential of these friendly bacteria.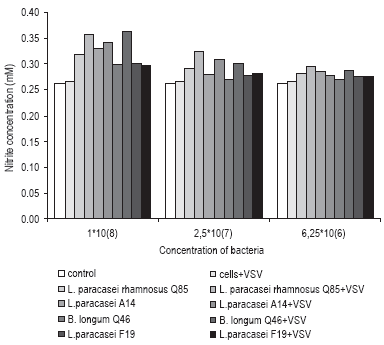
Figure 3. Production of NO in non-infected and VSV infected macrophages were assessed after 90 min pre-incubation of cell monolayers (6*106 cells/plate) with bacteria (2-fold dilutions from initial concentration of 1*108 bacteria/ml) at 37° C in atmosphere of 5% CO2. Following incubation, cells were carefully rinsed with PBS to remove excess of the bacteria and the plates were further incubated for 24h before VSV challenge. 24hrs after virus infection the NO assay was performed. Released NO in cell supernatant was measured by addition of Griess reagent. The A540 was measured, and the results were expressed, for each dilution, by the mean ratios (mM, ± SD) of absorbances in treated wells to those in control wells.
Conclusion and future perspectives
Can probiotic have a benefitial impact on the health status of farmed animals?
There is considerable interest in the utility of probiotic theraphy-the feeding of (live) non-pathogenic bacteria, originally derived from the alimentary tract, for disease treatment or health promotion. The microflora of the
gastrointestinal tract is essential for mucosal protection, for immune education and for metabolism of fecal residue. Physiological disturbances of these processes, when they occure, results from:
1) alteration of a microbial ecosystem, originally conserved by evolution;
2) reduces consumption of microorganisms with uniformed feeding;
3) invasion of pathogens.
Data support the use of proven probiotic organisms in prevention and treatment of flora-related gastrointestinal disorders. Therapeutic activity of probiotic bacteria can be due to competition with pathogens for nutrients and mucosal adherence, production of antimicrobial substances, and modulation of mucosal immune functions. Observed beneficial effect of these friendly bacteria were mainly been studied in In vivo trials on diverse animal models were results implicate on their ability of immunomodulation and protection against several emerging animal pathogens as Salmonella thyphimurium, Listeria and Campylobacter.
Results of our study furthermore implicate that probiotics can be used in protection against several virus caused infections in animals. Overall, it can be concluded that probiotics are most beneficial in health as preventive or therapeutic tool for farming animals.
References
Anderson’s A. http:// www.geocities .com/ artnscience/ peripheral-lt.html
Beausoleil HE, Labrie V, Dubreuil JD. Trypan blue uptake by chinese hamster ovary cultured epithelial cells: a cellular model to study Escherichia coli STb enterotoxin. Toxicon. 2002; 40: 185-191.
Bereswill S, Kist M. Recent development in Campylobacter pathogenesis. Curr. Opini. Infect. Dis., 2003; 16(5): 487-491.
Botiæ T., Jakobsen M., Weingertl H., Cenciè A. Antiviral activity of probiotic bacteria – a study in a cell culture model. IJFM, submitted.
Canil C, Rosenshine I, Ruchkowski S, Donnenberg MS, Kaper JB, Finlay BB, Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarised epithelial monolayers. Infect. Immun. 1993; 61: 2755-2762.
Ceylan A, Ertem M, Ilcin E, Ozekinci T. A special risk group for hepatitis E infection: Turkish agricultural workers who use untreated waste water fro irrigation. Epidemiol Infect. 2003; 131:753-756.
Chibber RM, Usmani MA, Al-Sibai MH, Should HEV infected mothers breast fed? Arch Gynecol Obstet. 2003.
Clancy R. Immunobiotics and the probiotic evolution. FEMS, Immunology and Medical Microbiology. 2003; 38: 9-12.
Clemente-Casares P, Pina S, Buti M, Jardi R, Martin M, Bofill-MasGirones R. Hepatitis E virus epidemiology in industrialized countries. Emerg Infect Dis.2003; 4:448-54.
Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastroinstestinal ecosystem: What we know and need to know from gnotobiology? MMBR. 1998; 62: 1157-1170.
Gradišnik L., Filipiè B., de Vaureix C., Lefevre F., La Bonnardiere C., Cenciè A. Establishment of a functional cell culture model of the pig small intestine.13th MEGAT Congress on “Alternatives to animal experimentation”, Linz, 2.6. - 4.6. 2006,. ALTEX, Altern. Tierexp., 2006, 23: 94.
Hostetter JM, Steadham EM, Haynes JS, Bailey TB, Cheville NF. Cytokine effects on maturation of the phagosomes containing Mycobacteria avium subspecies paratuberculosis in J774 cells. FEMS Immunol. and Med. Microbiol., 2002; 34: 127-134.
Juceviciene A, Vapalahti O, Laiskonis A, Ceplikiene J, Leinikki P. Prevalence of tick-borne-encephalitis virus antibodies in Lithuania. J Clin Virol. 2002; 25:23-27.
Juceviciene A, Vapalahti O, Laiskonis A, Ceplikiene J, Leinikki P. Prevalence of tick-borne-encephalitis virus antibodies in Lithuania. J Clin Virol. 2002; 25:23-27.
Heyman M. and Ménard S. Probiotic microorganisms: how they affect intestinal pathophysiology CMLS, Cell. Mol. Life Sci. 2002; 59: 1-15
Kalis CHJ., Collins MT, Hesslink JW, Barkema HW. Specificity of two tests for the early diagnosis of bovine paratuberculosis based on cellmediated immunity: the Johnin skin test and the gamma interferon assay. Vet. Microbiol., 2003; 97: 73-86.
Kernies S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997; 277: 949-952.
Krawczynski K, KamiliS, Aggarwal R. Global epidemiology and medical aspects of hepatitis E. Forum(Genova). 2001; 11:166-79.
McCluskie MJ, Davis HL. Mucosal immunization with DNA vaccines. Microbes Infect. 1999;1(9):685-98.
McCormik B. The use of transepithelial models to examine host-pathogen interactions. Curr.Opion. Microb. 2003; 6: 1-5.
Ogra PL, Faden H, Welliver RC.Vaccination strategies for mucosal immune responses. Clin Microbiol Rev. 2001 Apr;14(2):430-45.
Parrott DMV. 1976. The gut-associated lymphoid tissues and gastrointestinal immunity. A. Ferguson, and R. M. M. MacSween, eds. Immunological Aspects of the Liver and Gastrointestinal Tract 1. MTP Press, Lancaster.
Rastall RA, Gibson GR, Harsharnjit SG, Guarner F, Klaenhammer TR, Pot B, Reid G, Rowland IR, Sanders ME. 2005. Modulation of the microbial ecology of the human colon by probiotics, prebiotics and sybiotics to enhance human health: An overview of enabling science and potential applications. FEMS Microbial Ecology, 52: 145-152.
Rosenthal KL, Gallichan WS Challenges for vaccination against sexuallytransmitted diseases: induction and long-term maintenance of mucosal immune responses in the female genital tract. Semin Immunol. 1997 Oct;9(5):303-14.
Syzymanski CM, Logan SM, Linton D, Wren BW. Campylobacter – a tale of two protein glycoslation systems. Trends in Microbiol. 2003; 11: 233-238.
van Ginkel FW, Nguyen HH, McGhee JR Vaccines for mucosal immunity to combat emerging infectious diseases. Emerg Infect Dis. 2000 Mar-Apr;6(2):123-32.
Wassenaar TM, Blaser MJ. Pathophysiology of Campylobacter jejuni infections of humans. Microbes and Infection. 1999; 1:1023-1033.
Widdowson MC, Jaspers WJM, Van der Poel WHM, Verschoor F, de Roda Husman AM, Winter HLJ, Zaaijer HL, Koopmans M. Cluster of cases of acute hepatitis associated with hepatitis E virus infection acquired in the Netherlands. Clin Infect Diseas. 2003; 36:29-33.
Widdowson MC, Jaspers WJM, Van der Poel WHM, Verschoor F, de Roda Husman AM, Winter HLJ, Zaaijer HL, Koopmans M. Cluster of cases of acute hepatitis associated with hepatitis E virus infection acquired in the Netherlands. Clin Infect Diseas. 2003; 36:29-33.
Yazaki Y, Mizuo H, Takahashi M, Nishizawa T, Sasaki N, Gotanda Y, Okamoto H. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol. 2003. 84 : 2351-2357.
Authors: AVRELIJA CENCIČ 1,2, BIBIANA RUZO NOYA 1, TANJA BOTIC 1
1 University of Maribor, Faculty of Agriculture, Department of Microbiology, Biochemistry, Molecular Biology and Biotechnology, Vrbanska c. 30, 2000 Maribor, Slovenia
2 University of Maribor, Medical Faculty, Department for Biochemistry, Slomskov trg 15, 2000 Maribor, Slovenia.






.jpg&w=3840&q=75)