Evaluation of coronary dominance in pigs; a comparative study with findings in human hearts
Coronary dominance in swine has been poorly evaluated. The frequencies of each type of dominance have been described, but few details have been given as to the different expressions of each one. The aim of this study was to characterize coronary dominance in commercial breed swine. One hundred and fifty eight pig hearts were evaluated. The coronary arteries (CA) were infused with synthetic resin (Palatal 85% and Styrene15%) through the ostia after channeling. The coronary artery that gives origin to the posterior interventricular artery (PIA), and the site of termination of both the circumflex arteries (CXA), and left retroventricular branch (LRVB) were determined in order to establish the coronary dominance pattern. Right coronary dominance was found in 105 hearts (66.5%), and a balanced circulation in 53 specimens (33.5%). No dominance was observed for the left coronary artery in the hearts studied. The CXA ended on the posterior aspect of the left ventricle in 101 samples (64%) and on the crux cordis in 55 specimens (34.8%). In two specimens (1.3%) it ended as a left marginal artery. In all cases the PIA was a branch of the RCA, and was long in 105 hearts (66%), 55% of which corresponded to males and 45% to females, but this difference was not statistically significant (p=0.77). The AIA ended on the apex in 126 specimens (80%), 71 of which (56%) corresponded to males and 55 (44%) to females (p=0.74). Regarding right coronary dominance, subtype I was observed in 98 specimens (93.3%), subtype II in 5 cases (4.8%), whereas subtype III was observed in 2 hearts (1.9%). Knowing coronary dominance patterns and their irrigation territories is useful for training purposes based on the use of experimental and hemodynamic models with this animal species.
Keywords: pig, coronary dominance, coronary arteries, left dominance, balanced circulation.


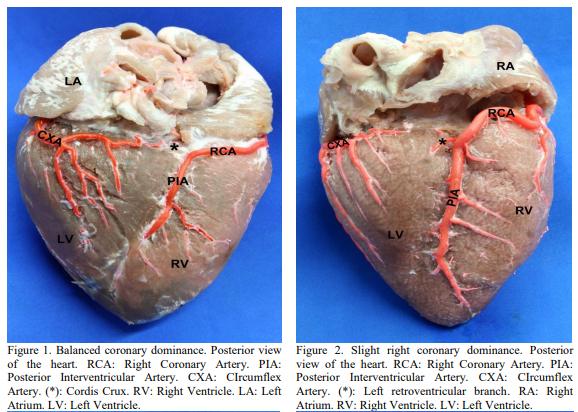
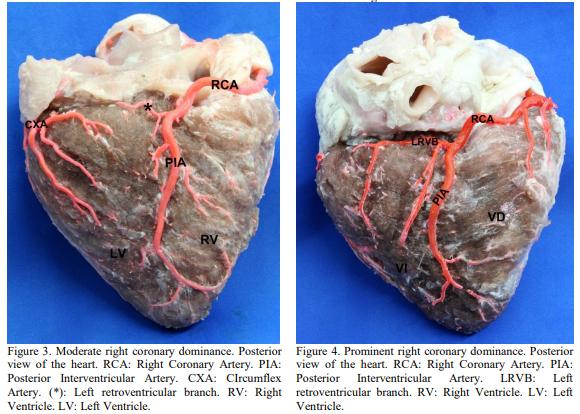
BALLESTEROS, L.E.; CORZO, E.G.; SALDARRIAGA, B. Determinación de la dominancia coronaria en población mestiza colombiana. Un estudio anatómico directo. Int. J. Morphol., v.25, p.483-491, 2007.
BALLESTEROS, L.E.; RAMIREZ, L.M.; QUINTERO, I.D. Right coronary artery anatomy: anatomical and morphometric analysis. Rev. Bras. Cir Cardiovasc., v.26, p.230-237, 2011.
BAPTISTA, C.A.; DIDIO, L.J.; PRATES J.C. Types of division of the left coronary artery and the ramus diagonalis of the human heart. Jpn. Heart. J., v.32, p.323-335, 1991.
BAPTISTA, C.A.; DIDIO, L.J.; TEOFILOVSKIPARAPID, G. Variation in length and termination of the right coronary artery in man. Jpn. Heart. J., v.30, p.789-798, 1989.
BAROLDI, G.; SCOMAZZONI, G. Coronary circulation in the normal and the pathologic heart. Office of the Surgeon General. Washington, DC: Dept of the Army, 1967.
BLUNK, J.N.; DIDIO, L.J.A. Types of coronary circulation in the human hearts. Ohio St. Med. J., v.67, p.596-607, 1971.
CAVALCANTI, J.S.; DE LUCENA OLIVEIRA, M.; PAIS E MELO, A.V. et al. Anatomic variations of the coronary arteries. Arq. Bras. Cardiol., v.65, p.489- 492, 1995.
CRICK, S.; SHEPPARD, M.; YEN HO, S. et al. Anatomy of the pig heart: comparisons with normal human cardiac structure. J. Anat., v.193, p.105-119, 1998.
DIDIO, L.J.; WAKEFIELD, T.W. Coronary arterial predominance or balance on the surface of the human cardiac ventricles. Anat. Anz., v.137, p.147-158, 1975.
HADZISELIMOVIC, H. Age characteristics of blood vessels of the human heart. Acta. Anat. (Basel), v.109, p.231-237, 1981.
ILIA, R.; ROSENSHTEIN, G.; WEINSTEIN J. et al. Left anterior descending artery length in left and right coronary artery dominance. Coron. Artery. Dis., v.12, p.77-78, 2001.
JAMES, T.N. Anatomy of the coronary arteries in health and disease. Circulation., v.32, p.1020-1033, 1965.
KAIMKHANI, Z.A.; ALI, M.M.; FARUQI, A.M. Pattern of coronary arterial distribution and its relation to coronary artery diameter. J. Ayub. Med. Coll. Abbottabad., v.17, p.40-43, 2005.
KALPANA, R.A. Study on principal branches of coronary arteries in humans. J. Anat. Soc. India., v.52, p.137-140, 2003.
KATO, T.; YASUE, T.; SHOJI, Y. et al. Angiographic diference in coronary artery of man, dog, pig and monkey. Acta. Pathol. Jpn., v.37, p.361- 373, 1987.
LÓPEZ, M.; RUIZ, G.; RAMÍREZ, M. et al. Cambios fisiológicos en cerdo de cirugía experimental para trasplante cardíaco. Investigación en Salud, v.6, p.11- 13, 2004.
LOUKAS, M.; CURRY, B.; BOWERS, M. et al. The relationship of myocardial bridges to coronary artery dominance in the adult human. Heart. J. Anat., v.209, p.43-50, 2006.
LUMB, G.; SINGLETARY, H. Blood supply to the atrioventricular node and bundle of hiis: a comparative study in pig, dog, and man. Grant H-5o63 from the National Institutes of Health, United States Public Health Service, v.41, p.65-75, 1962.
MARGARIS, N.G.; KOSTOPOULOS, K.G.; NERANTZIS, C.E. et al. Posterior right diagonal artery. An angiographic study. Angiology, v.48, p.73- 77, 1997.
MOUCHET, A. Les arteres coronaires du coeur chez l’Homme. 2nd ed. Maloine, Paris. 1993.
NERANTZIS, C.E.; PAPACHRISTOS, J.C.; GRIBIZI, J.E. et al. Functional dominance of the right coronary artery: incidence in the human heart. Clin. Anat., v.9, p.10-13, 1996.
ORTALE, J.R.; KEIRALLA, L.C.; SACILOTTO, L. The posterior ventricular branches of the coronary arteries in the human heart. Arq. Bras. Cardiol., v.82, p.468-472, 2004.
PENTHE, P.; BARA, J.A.; BLANC, J.J. Etude anatomique descriptive des gros troncs coronariens et des principales collaterales epicardiques. Nouv. Presse. Med., v.5, p.71- 75, 1976.
PESSA, C.J.; GOMES, W.J.; CATANI, R. et al. Anatomical relationships between the posterior mitral valve annulus and the coronary arteries. Implications to operative treatment. Braz. J. Cardiovasc. Surg., v.19, p.372-377, 2004.
POLACEK, P.; ZECHMEISTER, A. The occurrence and significance of myocardical bridges and loops on coronary arteries. University. J. E. Purkyne, v.10, p.125-131, 1958.
SAHNI, D.; JIT, I. Blood supply of the human interventricular septum in north-west Indians. Indian. Heart. J., v.42, p.161-169, 1990.
SAHNI, D.; KAUR, G.D.; JIT, H. et al. Anatomy and distribution of coronary arteries in pig in comparison with man. Indian J. Med. Res., v.127, p.564-570, 2008.
SCHLESINGER M.J. Relation of anatomic pattern to phatologic conditions of the coronary arteries. Arch. Pathol., v.30, p.403-415, 1940.
WEAVER, M.E.; PANTELY, G.A.; BRISTOW, J.D. et al. A quantitative study of the anatomy distribution of coronary arteries in swine in comparison with other animals and man. Cardiovasc. Res., v.20, p.907-917, 1986.
ZBIGNIEW, K.; MIKUSEK, J. Clinical and morphological studies on varieties of coronary vascularisation of diaphragmatic surface of human heart. Med. Sci. Monit., v.6, p.253-257, 2000.







