Antibiotics and their role in Brachyspira elimination programs
Published: February 18, 2014
By: Aaron J. Lower, DVM (Carthage Veterinary Service, Carthage, Illinois)
Introduction
With any bacterial elimination program, sanitation coupled with discontinuing contamination of the environment is fundamental to eradicating the organism from a premise. Site depopulation is the easiest way to discontinue contamination of the environment and has led to all in all out pig flow or depopulation/repopulation strategies. The decision to depopulate involves a variety of considerations including feasibility, economics, and site biosecurity. In situations where the production economics of continuing to populate a facility with infected animals versus depopulation are advantageous, antibiotic interventions are employed to assist with discontinuing contamination of the environment. This strategy increases the risk of failure in the elimination program and increases cost through antibiotic usage but is calculated to be offset by continued production and the economics of the situation. An increase in the laboratory diagnosis of strong beta hemolytic Brachyspira and associated lesions since 2006 coupled with the recent identification of B. hampsonii has reinvigorated the practitioners interest in this reemerging pathogen. Antibiotic selection and dosage play a key role in elimination of this pathogen.
Program
In large sow herds that are not depopulated, a structured and rigorous program with quality assurance checks must be employed to eliminate Brachyspira. Due to the labor requirements of the program, external labor and twenty four hour washing crews are often times warranted due to the considerable work load and scope of the project. This program requires excellent sanitation of the following areas:
1. Animal areas
a. Presoak areas with a detergent
b. Strict sanitation of flooring, side walls, ceiling, feed, and ventilation systems
c. Inspection and certification by veterinarian or upper management
d. Disinfection with quaternary/glutaraldehyde disinfectant with contact time of 10 minutes prior to moving animals
e. Removal of water nipples, cleaning and disinfection
f. Prevention of backsplash – use of plastic and tarps during pressure washing between clean and dirty areas
2. Animals
a. Washed prior to movement with particular attention to feet, legs and underline
b. Animals disinfected with iodine
3. Fomites
a. Danish changes between clean and dirty areas
b. McREBEL during site sanitation. No use of hot boxes, processing carts, etc.
4. Water source
a. Inspection of holding tank, ensure no rodent or bird exposure.
5. Rodent/pest control
a. Bait stations every 50 feet around the perimeter, checked twice weekly.
b. Bait stations installed in the barn attics, checked weekly.
c. Grass and weeks mowed and sprayed around the barns. Intact rock barrier surrounds the perimeter of all barns.
d. Implementation of The Rodent Index System (adapted from Penn State Cooperative Extension) to quantify rodent activity.
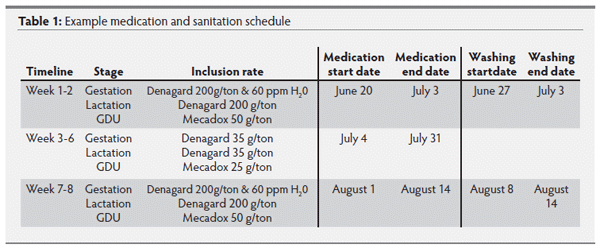
e. Boric acid applied to all electric boxes and areas where cockroaches congregate. Creep dividers in farrowing house filled with foam spray to eliminate cockroach habitat. (Table 1)
Antibiotics commonly used for treatment of Brachyspira include tiamulin, carbadox, tylosin, lincomycin, gentamicin, valnemulin, and salinomycin. Of those tiamulin and carbadox are most commonly utilized in the United States. Brachyspira MIC values to tylosin are intermediate and high MIC values to lincomycin and gentamicin provide concern of resistance to these antibiotics.5 Valnemulin is not approved for use in the United States and salinomycin is not labeled for use in swine.
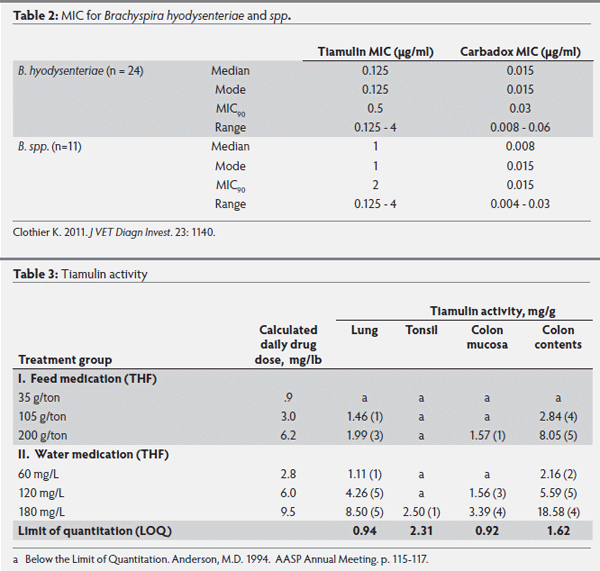
MIC data for the pleuromutalin drugs, valnemulin and tiamulin, suggest that antibiotic resistance patterns in US isolates of Brachyspira are not developing as quickly as what has been described in other countries. Carbadox and salinomycin MIC levels are at the low end of the range tested and expected to be effective. The MIC90 of Brachyspira hyodysenteriae and spp is 0.03 and 0.015 respectfully. Administration of carbadox at 50 g/ton provides colonic activity at 4 mg/g (Dwain Guggenbiller. personal communication. June 2012).
When a herd is infected with Brachyspira hampsonii, MIC patterns are generally elevated compared to Brachyspira hyodysenteriae. Susceptibility testing in 2011, examined MIC patterns of 11 North American sourced Brachyspira spp. isolates. 91% (9/11) of these isolates exhibited strong beta hemolysis. After the study was published, these isolates were sequenced and mostly identified to be B. hampsonii (Eric Burrough, personal communication, June 2012). The median MIC values from B. hampsonii are 8 time higher and the MIC90 4 times higher than isolates of B. hyodysenteriae tested in this study (n = 24). Tiamulin colonic contents activity is outlined in Figure C with the MIC90 setting a target of 2 for B. hampsonii. Ad libitum feed intake in lactation results in a calculated daily drug dose of 2.4 mg/lb (500 lb sow with 12 lb per day intake – 200 g/ ton tiamulin). This is slightly less than the published daily dosage of 3.0 mg/lb which supplies activity in colon contents at 2.84 mg/g7 but is estimated to supply tiamulin activity of > 2 mg/g (Keith Erlandson, personal communication, June 2012). Restricted feed intake in gestation results in a calculated daily drug dose of 0.9 mg/lb (500 lb sow with 5 lb per day intake – 200 g/ton tiamulin) which results in colonic content concentrations below the limit of quantification (<1.62 mg/g). Since this is less than our target MIC value of 2, supplemental tiamulin must be administered via the drinking water. Additional dosage of tiamulin at 60 ppm provides a calculated daily drug dose of 2.27 mg/lb7 (500 lb sow with 5 gal per day intake). Total combined dosage is estimated to be ~3.27 mg/lb which is estimated to provide tiamulin activity of >2 mg/g in the colon contents (Keith Erlandson, personal communication, June 2012). (Tables 2 and 3)
References
Burrough E. Brachyspira-Associated Colitis. ISU Swine Disease Conference proceedings. 2012. pp 43– 47.
Chander Y, Primus A, Oliveira S, et al. Phenotypic and molecular characterization of a novel strongly hemolytic Brachyspira species, provisionally designated “Brachyspira hampsonii”. JVetDiagn Invest 2012; 24:903–910.
Gebhart C. Efficacy of Synergize against Brachyspira hyodysenteriae. Preserve International report. Received by email June 2012.
Martin G. The Rodent Index System. Penn State Extension – poultry. www.personal.psu.edu/gpm10/Rodent_Index11.pdf. Accessed on July 4, 2012.
Clothier K, Kinyon J, Frana T, Naberhaus N, Bower L, Strait E, Schwartz K. Species characterization and minimum inhibitory concentration patterns of Brachyspira species isolates from swine with clinical disease. 2011. J VET Diagn Invest. 23: 1140.
Straw B, Zimmerman J, D’Allaire S, Taylor D. Diseases of Swine; 9th Edition. Swine Dysentery pp. 785 – 805. Blackwell Publishing, 2006.
Content from the event:
Authors:
Carthage Veterinary Service, Ltd.
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.





