Simultaneous determination of mycotoxins in peruvian purple maize
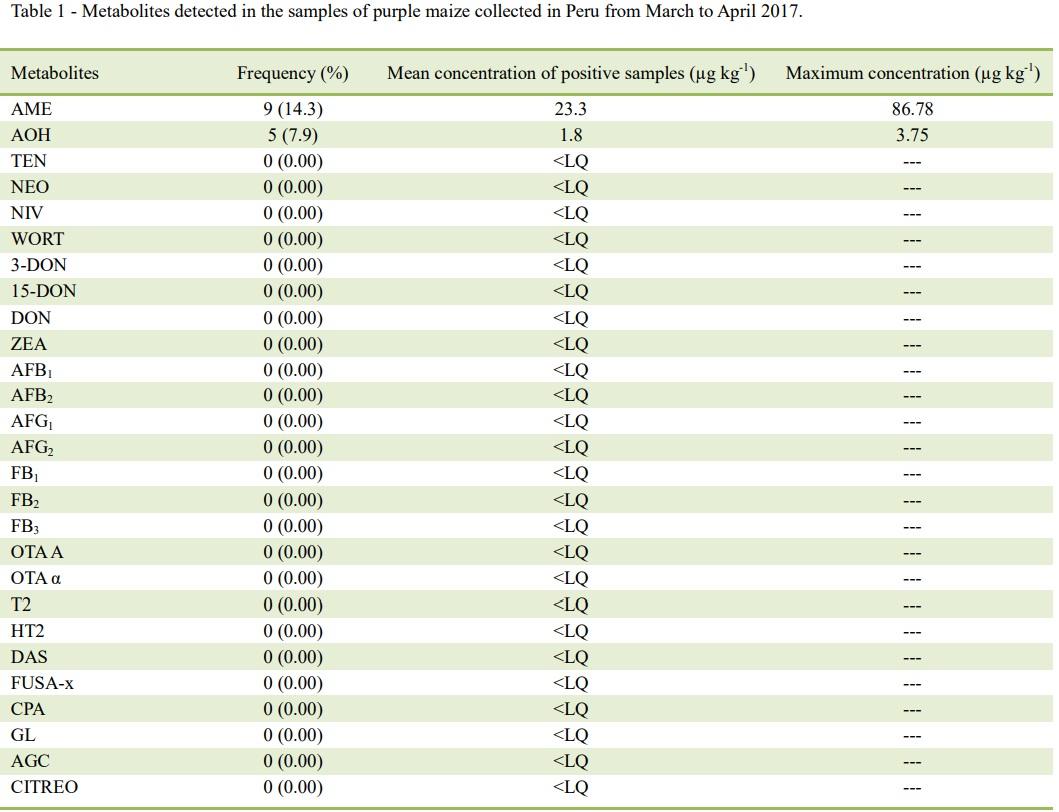
WORT=wortmannin, 3DON=3-acetyl deoxynivalenol, 15DON=15-acetyl deoxynivalenol, DON=deoxynivalenol, ZEA=zearalenone,
AFB1=aflatoxin B1, AFB2=aflatoxin B2, AFG1=aflatoxin G1, AFG2=aflatoxin G2, FB1=fumonisin B1, FB2=fumonisin B2, FB3=fumonisin
B3, OTA A=ochratoxin A, OTA α=ochratoxin α, T2=T-2 toxin, HT2=HT-2 toxin, DAS=diacetoxiscirpenol, FUSA-x=fusarenon x,
CPA=cyclopiazonic acid, GL=gliotoxin, AGC=agroclavin, CITREO=citreoviridin.
ADEYEYE, S.A.O. Aflatoxigenic fungi and mycotoxins in food: a review. Critical Reviews in Food Science and Nutrition, p.1-13.
2019. Available from: <https://www.tandfonline.com/doi/abs/10.
1080/10408398.2018.1548429?journalCode=bfsn20>. Accessed:
Feb. 5, 2019. doi: 10.1080/10408398.2018.1548429.
ARROYO, J. et al. Reducción del colesterol y aumento de la capacidad antioxidante por el consumo crónico de maíz morado (Zea mays L) en ratas hipercolesterolémicas. Revista Peruana de
Medicina Experimental y Salud Publica, v.24, p.157-162, 2007.
Available from: <http://www.scielo.org.pe/scielo.php?script=sci_ arttext&pid=S1726-46342007000200010&nrm=iso>. Accessed
Jan. 03, 2019.
BELHASSEN, H. et al. Zearalenone and its metabolites in urine and breast cancer risk: A case-control study in Tunisia. Chemosphere, v.128, p.1-6, 2015. Available from: <https://www.ncbi.nlm.nih. gov/pubmed/25602441>. Accessed: Jan. 03, 2019. doi: 10.1016/j. chemosphere.2014.12.055.
BOUTRIF, E.; CANET, C. Mycotoxin prevention and control FAO programmes. v.6, p.681-694, 1998. Available from: <https://www. revmedvet.com/artdes-us.php?id=143>. Accessed: Jan. 03, 2019.
BRASIL. Agência Nacional de Vigilância SanitáriaANVISA. Resolução da Diretoria Colegiada – RDC nº 7, de
18 de fevereiro de 2011 que dispõe sobre limites máximos tolerados (LMT) para micotoxinas em alimentos. Diário Oficial da União, Brasília, DF, 2011.
EFSA: Panel on contaminants in the food chain: scientific opinion on the risk for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J 2011, 9:2407.
HAJNAL, E.J. et al. Possibility of Alternaria toxins reduction by extrusion processing of whole wheat flour. Food Chemistry, v.213 p.784-790, 2016. Available from: <https://www.ncbi.nlm.nih.gov/ pubmed/27451248>. Accessed: Feb. 01, 2019. doi: 10.1016/j. foodchem.2016.07.019.
HUSSEIN, S.H.; BRASSEL, M. Toxicity, metabolism and impact of mycotoxins on human and animals. Toxicology, v.167, p.101-
134, 2001. Available from: <https://www.ncbi.nlm.nih.gov/ pubmed/11567776>. Accessed: Feb. 04, 2019.
JAMES, A.; ZIKANKUBA, V.L. Mycotoxins contamination in maize alarms food safety in sub-Sahara Africa. Food Control, v.90, p.372-381, 2018. Available from: <https://www.sciencedirect. com/science/article/pii/S0956713518301257>. Accessed: Feb. 03,
2019. doi: 10.1016/j.foodcont.2018.03.018.
KROUT-GREENBERG, N.D. et al. Preliminary study to assess mycotoxin concentrations in whole corn in the California feed supply. Journal Dairy Science, v.96, p.2705-2712, 2013.
Available from: <https://www.sciencedirect.com/science/article/ pii/S0022030213001318>. Accessed: Jan. 29, 2019. doi: 10.3168/ jds.2012-5957.
LEE, H.B. et al. Alternaria in Food: ecophysiology, mycotoxin production and toxicology. Mycobiology, v.43, p.93-106, 2015.
Available from: <https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC4505009/>. Accessed: Feb. 04, 2019. doi: 10.5941/
MYCO.2015.43.2.93.
MORENO, E.C. et al. Co-occurrence of mycotoxins in corn samples from the Northern region of Paraná State, Brazil. Food
Chemistry, v.116, p.220-226, 2009. Available from: <https:// www.sciencedirect.com/science/article/pii/S0308814609002258>.
Accessed: Feb. 05, 2019. doi: 10.1016/j.foodchem.2009.02.037.
MUNKVOLD, G.P. et al. Mycotoxins in Corn: Occurrence, Impacts, and Management. Corn, 235-287, 2019. Available from: <https:// www.sciencedirect.com/science/article/pii/B9780128119716000097>.
Accessed: Feb. 01, 2019. doi:10.1016/b978-0-12-811971-6.00009-7.
PATRIARCA, A.; PINTO, V.F. Alternaria. Reference Modulein
Food Science. Elsevier, 2018, 1-8. Available from: <https://www. researchgate.net/publication/325936280_Alternaria>. Accessed:
Feb. 05, 2019. doi: 10.1016/B978-0-08-100596-5.22572-9>.
SERRANO, A.B. Co-occurrence and risk assessment of mycotoxins in food and diet from Mediterranean area. Food Chemistry, v.135, p.423-429, 2012. Available from: <https://www.sciencedirect. com/science/article/pii/S0308814612005304>. Accessed: Feb. 03,
2019. doi: 10.1016/j.foodchem.2012.03.064.
TIEMANN, U. et al. The mycotoxins alternariol and alternariol methyl ether negatively affect progesterone synthesis in porcine granulosa cells in vitro. Toxicology Letters, v.186, p.139-
145, 2009. Available from: <https://www.ncbi.nlm.nih.gov/ pubmed/19429235>. Accessed: Feb. 06, 2019. doi: 10.1016/j. toxlet.2009.01.014.
VEJDOVSZKY, K. et al. Synergistic estrogenic effects of fusarium and alternaria mycotoxins in vitro. Arch toxicol, v.9, p.1447-1460, 2017. Available from: <https://www.ncbi.nlm.nih. gov/pubmed/27401186>. Accessed: Jan. 27, 2019. doi: 10.1007/ s00204-016-1795-7.