Comparison of Two Inoculation Methods for Evaluating Corn for Resistance to Aflatoxin Contamination
Published: February 18, 2009
By: Paul M. Buckley, W. Paul Williams and Gary L. Windham - Mississippi State University, Mississippi Agricultural & Forestry Experiment Station (Bulletin 1148)
Aflatoxin, produced by the fungus Aspergillus flavus, is a naturally occurring toxin in corn and the most potent carcinogen found in nature (Castegnaro and McGregor 1998, Park and Liang 1993). Dietary exposure to aflatoxin is one of the major causes of hepatocellular carcinoma, the fifth most common cancer in the world (Wild and Hall 2000). The U.S. Food and Drug Administration limits the sale of grain with aflatoxin levels exceeding 20 parts per billion (ppb) (Park and Liang 1993), and contaminated grain is restricted from interstate commerce.
Aflatoxin was first recognized as a major problem in the Southeastern U.S. in the 1970s. In 1977, more than 90% of the corn produced in the Southeast was contaminated with aflatoxin, and aflatoxin levels exceeded 20 ppb in 90% of the samples evaluated in Georgia (McMillian et al. 1978, Zuber et al. 1979). Aflatoxin has remained a chronic problem in the Southeast, reaching devastating proportions in 1998 when losses to aflatoxincontaminated corn in Arkansas, Louisiana, Mississippi, and Texas were estimated at $85 to $100 million. Differences in aflatoxin contamination among years and locations have been attributed to insect damage or heat, drought, and other weather-related factors (Widstrom et al. 1990).
Plant resistance is generally considered the best strategy for reducing or eliminating aflatoxin contamination, but commercial hybrids with adequate levels of resistance to aflatoxin are not currently available (Widstrom 1996, Windham and Williams 1999). Following the devastating aflatoxin epidemic of 1998, several publicly supported corn breeding programs in the South either initiated or expanded research on aflatoxin. A major component of this research is the identification and development of corn germplasm that can be used in the production of aflatoxin-resistant corn hybrids. Creating a situation that maximizes the opportunity for a uniform A. flavus infection of developing ears is essential to successfully differentiating among corn genotypes with different levels of resistance to aflatoxin contamination. Only then can genotypic differences associated with aflatoxin resistance be recognized. Various methods for inoculating corn with A. flavus have been used (Windham et al. 2003).
The side-needle technique has been used as the primary method of inoculation at Mississippi State for almost 20 years (Zummo and Scott 1989). Although effective, the technique is labor intensive and wounds the developing ear. Wounding of the ear provides a pathway for the fungus to enter the ear. Since 1998, efforts to develop a reliable inoculation technique that requires less labor and does not cause physical injury to the ear have been under way. Using multiple applications of an A. flavus spore suspension to developing ears using a backpack sprayer is one good method. The objectives of this study were to compare the side-needle and backpack- sprayer methods.
Experiments were conducted at the R.R. Foil Plant Science Research Center at Mississippi State University from 1999 to 2002 to compare side-needle and backpacksprayer methods of inoculating corn with A. flavus. Each experiment consisted of four single- cross hybrids planted in single-row plots, approximately 4 meters long spaced 1 meter apart, in a randomized complete block design with four replications per inoculation method. Two hybrids - Mp339 x SC212m and GA209 x SC212m - were selected for susceptibility to aflatoxin contamination. The other two hybrids - Mp313E x Mp420 and Mo18W x Mp313E - were selected for their resistance. In 1999 and 2000, one experiment was conducted. In 2001 and 2002, experiments were conducted at two sites, the second site being one that is prone to drought stress.
Aflatoxin was first recognized as a major problem in the Southeastern U.S. in the 1970s. In 1977, more than 90% of the corn produced in the Southeast was contaminated with aflatoxin, and aflatoxin levels exceeded 20 ppb in 90% of the samples evaluated in Georgia (McMillian et al. 1978, Zuber et al. 1979). Aflatoxin has remained a chronic problem in the Southeast, reaching devastating proportions in 1998 when losses to aflatoxincontaminated corn in Arkansas, Louisiana, Mississippi, and Texas were estimated at $85 to $100 million. Differences in aflatoxin contamination among years and locations have been attributed to insect damage or heat, drought, and other weather-related factors (Widstrom et al. 1990).
Plant resistance is generally considered the best strategy for reducing or eliminating aflatoxin contamination, but commercial hybrids with adequate levels of resistance to aflatoxin are not currently available (Widstrom 1996, Windham and Williams 1999). Following the devastating aflatoxin epidemic of 1998, several publicly supported corn breeding programs in the South either initiated or expanded research on aflatoxin. A major component of this research is the identification and development of corn germplasm that can be used in the production of aflatoxin-resistant corn hybrids. Creating a situation that maximizes the opportunity for a uniform A. flavus infection of developing ears is essential to successfully differentiating among corn genotypes with different levels of resistance to aflatoxin contamination. Only then can genotypic differences associated with aflatoxin resistance be recognized. Various methods for inoculating corn with A. flavus have been used (Windham et al. 2003).
The side-needle technique has been used as the primary method of inoculation at Mississippi State for almost 20 years (Zummo and Scott 1989). Although effective, the technique is labor intensive and wounds the developing ear. Wounding of the ear provides a pathway for the fungus to enter the ear. Since 1998, efforts to develop a reliable inoculation technique that requires less labor and does not cause physical injury to the ear have been under way. Using multiple applications of an A. flavus spore suspension to developing ears using a backpack sprayer is one good method. The objectives of this study were to compare the side-needle and backpack- sprayer methods.
Experiments were conducted at the R.R. Foil Plant Science Research Center at Mississippi State University from 1999 to 2002 to compare side-needle and backpacksprayer methods of inoculating corn with A. flavus. Each experiment consisted of four single- cross hybrids planted in single-row plots, approximately 4 meters long spaced 1 meter apart, in a randomized complete block design with four replications per inoculation method. Two hybrids - Mp339 x SC212m and GA209 x SC212m - were selected for susceptibility to aflatoxin contamination. The other two hybrids - Mp313E x Mp420 and Mo18W x Mp313E - were selected for their resistance. In 1999 and 2000, one experiment was conducted. In 2001 and 2002, experiments were conducted at two sites, the second site being one that is prone to drought stress.

Figure 1. With the side-needle technique, an A. flavus spore suspension was injected into the side of the ear using a tree-marking gun fitted with a 14-gauge needle.
With the backpack-sprayer method, inoculations were initiated for all plots approximately 7 days after silk emergence in the earliest maturing hybrids. Weekly, for 5 weeks, the husks and silks of ears and ear shoots were sprayed with a spore suspension to which a spreader sticker had been added using a backpack sprayer (Figure 2). Approximately 300 million conidia per plant were applied with each application.
In 2001 and 2002, two additional experiments were conducted to compare aflatoxin levels when inoculations were begun at 7, 14, 21, or 35 days after midsilk. Treatments included one, three, or five inoculations. Two commercial hybrids, DK683 and TV2100, were planted each year. The experimental design was a randomized complete block with six replications with treatments arranged as a split plot. Hybrids were assigned to main plots and inoculations to subplots.

Figure 2.A backpack sprayer was used to apply an A. flavus spore suspension to the silks and husks of developing ears weekly for 5 weeks.
For all experiments, ears were hand-harvested about 60 days after midsilk and dried at 38°C for 7 days. The dried ears were shelled, and the grain was ground with a Romer mill. Aflatoxin contamination was determined using the Vicam Aflatest (Watertown, Massachusetts). Data were subjected to analysis of variance, and means were compared by Fisher’s Protected LSD (Steel and Torrie 1980).
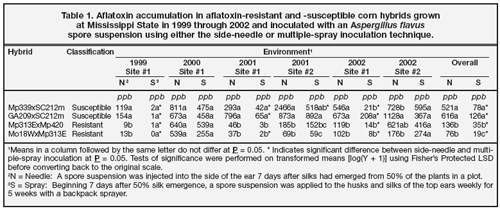
The side-needle and backpack-spray methods were first compared in 1998 when aflatoxin contamination was extremely high (Windham and Williams 1999). Among a group of 45 commercial corn hybrids, aflatoxin levels ranged from 529 to 11,936 ppb with the side-needle inoculation and from 910 to 8,100 ppb with the spray inoculation. In this investigation, levels of aflatoxin contamination varied among years and sites (Table 1). Aflatoxin contamination was higher in 2000 and at Site 2 in 2001 and 2002 than in the other environments. In these three environments, differences between the two methods were not statistically significant except for Mp339 x SC212m in 2001. Differences among hybrids were not statistically significant with either method in 2000. In 2002 at Site 2, neither method effectively differentiated between the resistant and susceptible hybrids.
Although both inoculation methods failed to differentiate between aflatoxin-resistant and aflatoxin-susceptible hybrids in some environments, differences between hybrids were significant with both techniques when data were pooled over environments. Over all environments, aflatoxin levels were significantly higher with the side-needle inoculation than the spray inoculation, though the results indicate that either method could be used effectively to screen for aflatoxin resistance. Germplasm should, however, be evaluated in more that one environment regardless of the method of inoculation.

Figure 3. The ears on the left were inoculated using the side-needle technique. The ears on the right were inoculated using the multiplespray technique and infested with southwestern corn borer. Feeding by southwestern corn borer provided sites for A. flavus to enter the ear.
The spray inoculation technique has proved useful in combination with southwestern corn borer, Diatraea grandiosella Dyar, infestation to investigate the association between southwestern corn borer damage and aflatoxin accumulation (Williams et al. 2002a, b). Results of these investigations indicated that in environments conducive to aflatoxin accumulation, southwestern corn borer damage increased aflatoxin contamination.
The need for an inoculation method that does not wound the ear was demonstrated when pairs of transgenic Bt and non-Bt conventional hybrids were infested with southwestern corn borer and inoculated with either the spray or side-needle technique (Williams et al. 2002b). When inoculated with the side-needle technique, aflatoxin contamination was high in both Bt and non-Bt hybrids. When ears were inoculated with multiple sprays, aflatoxin contamination was significantly greater in non- Bt than Bt hybrids, whether or not plants were also infested with southwestern corn borer. The wounding of kernels with the side-needle technique provided a point of entry for the fungus into the ear, and the resulting fungal growth on the ear frequently appeared similar to that observed around insect feeding sites when the spray inoculation technique was used in combination with southwestern corn borer infestation (Figure 3). It was only with the nonwounding spray technique that the benefits of Bt hybrids in reducing aflatoxin contamination were realized.
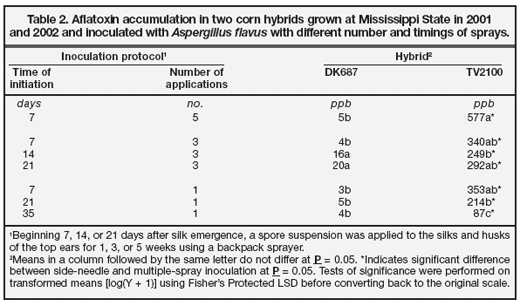
The results of experiments conducted in 2001 and 2002 to compare aflatoxin accumulation following inoculations using one, three, or five sprays beginning at 7, 14, 21, or 35 days after midsilk are presented in Table 2. Aflatoxin contamination levels were relatively low regardless of the timing of inoculations. Aflatoxin levels were significantly higher for TV2100 than DK687 for all inoculation protocols. The highest level of aflatoxin resulted from five applications of inoculum to TV2100. Three applications begun at 7 or 21 days after midsilk or one application at 7 days after midsilk resulted in aflatoxin levels that were not significantly lower, however. The lowest level of aflatoxin contamination occurred when inoculum was applied only once at 35 days after silk emergence.
Click here to enlarge the image
CONCLUSIONS
The side-needle inoculation method produces significantly higher levels of aflatoxin contamination than the backpack-spray technique in most environments. Both methods are generally effective in differentiating between resistant and susceptible hybrids. However, the spray technique tends to be less effective in low-stress environments where aflatoxin levels are low. Because the side-needle technique causes damage similar to that caused by insect feeding, the spray technique is better suited for investigations of the role of insects in aflatoxin contamination.
When using the spray technique, fewer than five applications could be used. The application made at 35 days after midsilk is probably least critical to success. One advantage of the spray technique is that data on silk emergence are not essential for all plots. Once spraying is initiated in an experiment, timing of inoculations is no longer linked to silking dates of individual plots. All plots are sprayed each week. To reduce the time needed to apply inoculum using the backpack spray technique, a backpack sprayer could be adapted to spray two rows simultaneously (Figure 4). High clearance sprayers could be used to further reduce application time for large experiments. When genotypes under investigation vary in maturity, five applications may compensate for variations among hybrids in maturity. The most appropriate method of inoculation depends on the objectives of the experiment.

Figure 4. A backpack sprayer can be adapted to apply a spore suspension to two rows simultaneously.

Figure 5.Feeding by southwestern corn borer provided sites for A. flavus to enter the ear.
REFERENCES
Castegnaro, M., and D. McGregor. 1998. Carcinogenic risk assessment of mycotoxins. Rev. Med. Vet. 149: 671- 678.
McMillian, W.W., D.M. Wilson, and N.W. Widstrom. 1978. Insect damage, Aspergillus ear mold, and aflatoxin contamination in south Georgia corn fields in 1977. J. Environ. Qual. 7: 564-56
Park, L.D., and Liang, B. 1993. Perspectives on aflatoxin control for human food and animal feed. Trends Food Sci. Technol. 4: 334-342.
Steel, R.D.G., and J.H. Torrie. 1980. Principles and procedures of statistics. McGraw-Hill. New York.
Widstrom, N.W. 1996. The aflatoxin problem with corn grain. Adv. Agron. 56: 219-280.
Widstrom, N.W., W.W. McMillian, R.W. Beaver, and D.M. Wilson. 1990. Weather-associated changes in aflatoxin contamination in preharvest maize. J. Prod. Agric. 3: 196-199.
Wild, C.P., and A.J. Hall. 2000. Primary prevention of hepatocellular carcinoma in developing countries. Mutat. Res. 462: 381-393.
Williams, W.P., P.M. Buckley, and G.L. Windham. 2002a. Southwestern corn borer (Lepidoptera: Crambidae) damage and aflatoxin accumulation in maize. J. Econ. Entomol. 95: 1049-1053.
Williams, W.P., G.L. Windham, P.M. Buckley, and C.A. Daves. 2002b. Aflatoxin contamination in conventional and transgenic corn hybrids infested with southwestern corn borer (Lepidoptera: Crambidae). J. Agric. Urban Entomol. 19: 227-236.
Windham, G.L., and W.P. Williams. 1999. Aflatoxin accumulation in commercial corn hybrids in 1998. Miss. Agric. And Forestry Exp. Stn. Res. Rep. 22(8). Mississippi State, MS.
Windham, G.L., W.P. Williams, P.M. Buckley, and H.K. Abbas. 2003. Inoculation techniques used to quantify aflatoxin resistance in corn. J. Toxicol. Toxin Reviews. 22: 313-325.
Zuber, M.S., O.H. Calvert, E.B. Lillehoj, and W.F. Kwolek. 1979. Status of aflatoxin problem in corn. J. Environ. Qual. 8: 1-5.
Zummo, N., and G.E. Scott. 1989. Evaluation of field inoculation techniques for screening maize genotypes against kernel infection by Aspergillus flavus in Mississippi. Plant Dis. 73: 313-316.
Authors:
Paul M. Buckley, Agronomist USDA-ARS Corn Host Plant Resistance Research Unit, Mississippi State
W. Paul Williams, Supervisory Research Geneticist USDA-ARS Corn Host Plant Resistance Research Unit, Mississippi State
Gary L. Windham, Research Plant Pathologist USDA-ARS Corn Host Plant Resistance Research Unit, Mississippi State
Paul M. Buckley, Agronomist USDA-ARS Corn Host Plant Resistance Research Unit, Mississippi State
W. Paul Williams, Supervisory Research Geneticist USDA-ARS Corn Host Plant Resistance Research Unit, Mississippi State
Gary L. Windham, Research Plant Pathologist USDA-ARS Corn Host Plant Resistance Research Unit, Mississippi State
Related topics:
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.





.jpg&w=3840&q=75)