Determination of fungal microbiota and mycotoxins in brewers grain used in dairy cattle feeding in the State of Bahia, Brazil
Published: December 28, 2007
By: Monica M.S. Simas, Mariana B. Botura, Benedito Correa, Myrna Sabino, Carlos A. Mallmann, Thereza C.B.S.C. Bitencourt, Maria J.M. Batatinha
© 2005 Elsevier Ltd. All rights reserved.
Keywords: Fungi; Barley; Mycotoxins
1. Introduction
The dairy cattle herd of the State of Bahia consists of 1,508,904 heads and produces 752,026,000 l of milk/year, corresponding to 3.5% of the national production (IBGE, 2003). The expansion of intensive and semi-intensive systems for the rearing of these animals has increased the need for storage of feed used during prolonged dry periods. The use of agroindustrial residues as a food supplement for dairy cattle plays a signiWcant economic role due to the availability and versatility of these materials. Brewers grain are one of the most frequently used by-products mainly because of the proximity between breweries and dairy properties in this region, which reduces the cost of transportation of this material.
The microregions in which these dairy herds are reared are characterized by high temperatures, reaching up to 38 °C, a mean annual rainfall above 1000mm and relative air humidity greater than 70% (Anuário, 2002). These climatic conditions, together with the inadequate storage of brewers grain in the farms and the composition of this substrate, provide the ideal conditions for fungal development.
Various fungi produce mycotoxins, chemically diverse secondary metabolites that can be harmful both to human and animal health (CAST, 2003; Fink-Gremmels, 1999). Evidence shows the participation of these toxic agents in the etiology of serious pathological processes in production animals associated with animal feeds, including barley, and this kind of contamination has been the subject of intensive scientific investigation both in Brazil and in diVerent parts of the world (Kim, Kang, Lee, Lee, & Yoshizawa, 1993; Mallmann, Santurio, & Dilkin, 1999). Aflatoxins and ochratoxins are among the various mycotoxins identified that play an important role in animal health.
Because of the marked risk for animal health and productivity due to exposure to mycotoxin, continuous studies regarding the monitoring of these toxins in products to be used as animal feed are being performed worldwide.
In Brazil, most reports on the contamination of food products by mycotoxins refer to the south and southeast regions of the country, and mainly indicate the presence of aflatoxins, fumonisins, zearalenone, ochratoxin A and deoxynivalenol (Rodriguez-Amaya & Sabino, 2002). In view of the scarcity of information regarding the occurrence of mycotoxins in animal feed in the State of Bahia, the aim of the present study was to investigate fungal microbiota and the presence of aflatoxins and ochratoxins in brewers grain used as dairy cattle feed in this state.
2. Material and methods
2.1. Sample collection
Brewers grain samples were collected in Wve farms (A, B, C, D and E) located in the cities of the “recôncavo baiano” (exceptionally fertile region on the coast of the State of Bahia, Brazil). These properties were selected because they have the largest concentration of dairy cattle and constantly use this material as animal feed. In each property, four samples were collected every three months over a period of one year, in a total of 80 samples. The mean period the product remained in the storage tanks was considered the determining factor for sample collection.
A questionnaire regarding sanitary and nutritional management of the herd, as well as storage conditions and form of use of brewers grain, was applied during the visits to the farms.
2.2. Determination of the moisture content of the samples
The moisture content of brewers grain samples was determined according to the method by Silva (1991). Samples were previously weighed and dried at 105 °C until they reached a constant weight. Moisture content of each sample was determined by the difference between dry weight and initial weight.
2.3. Isolation of mycoflora (Swanson, Busta, Peterson,& Johnson, 1992)
Ten grams of each brewers grain sample was diluted in 90 ml sterile distilled water to obtain a 10-1 dilution. Using this stock solution, serial dilutions up to 10-4 were then prepared with the same diluent. Aliquots of 1ml of each dilution were plated on Petri dishes containing 15ml potato dextrose agar supplemented with 100 µg/ml chloramphenicol, and the plates were incubated at 25 °C for 5 days. Filamentous fungi were identified at the genus level (Nelson, Tousoun, & Marasas, 1983; Raper & Fennell, 1965).
2.4. Determination of mycotoxins in brewers grain
2.4.1. Total aflatoxins (Aflatest Manual, 2002)
Fifty grams of each sample were homogenized in a blender with 100ml methanol:water (80:20) and 5 g NaCl for 1min. After filtration, a 10-ml aliquot was completed to a volume of 50 ml with distilled water. The sample was then filtered a second time using microfiber paper and 10ml of this filtrate was applied to an immunoaffinity column (Aflatest®, Vicam). After passage of the sample, the column was washed twice with 10ml distilled water and then with 1ml methanol to elute aflatoxins bound to monoclonal antibodies. The Aflatest® agent used in the development was added to the eluate and aflatoxin content was read with a fluorimeter (series 4, Vicam). Detection limit of the method was 1 µg/kg.
2.4.2. Ochratoxins (Ochratest Manual, 2002)
For analysis of ochratoxins, 50 g of each sample were homogenized in a blender with 100ml methanol:water (80:20) for one minute. After Wltration, a 10-ml aliquot was completed to a volume of 50ml with distilled water. This solution was then Wltered a second time using microfiber paper and 10ml of this filtrate was applied to an immunoaffinity column (Ochratest®, Vicam). After passage of the sample, the column was washed with 10ml ochratoxin buffer solution and 10ml distilled water. After that, 1.5ml Ochratest® solution was used to elute ochratoxin bound to monoclonal antibodies. This eluate was immediately read using a fluorimeter (series 4, Vicam). Detection limit of the method was 2 µg/kg.
2.4.3. Quality control of the analyses
Tests were performed in triplicate to validate the protocols used in this study. In order to do this, confirmed negative samples were contaminated with a known amount of each mycotoxin standard (Sigma). The confirmation of negative samples were obtained through HPLC method at the Lamic (Laboratory of Mycotoxins, University of Santa Maria, Brazil) (Mallmann et al., 2000). After extraction, percentile of recovery was calculated as the difference between the fluorimetric value obtained for the test sample and the initial contamination of the same sample.
Recovery was 89.58% (cv%=8.97) and 66.66% (cv%= 35.34) for aflatoxins and ochratoxin A, respectively.
2.5. Climatological data
Climate information regarding mean, minimum and maximum temperature, mean rainfall and relative air humidity on the properties studied was provided by the National Institute of Meteorology of the State of Bahia (INMET-BA). The period of storage of brewers grain in the farms was the determining factor in the calculation of the mean climatological data used here.
2.6. Statistical analysis
The influence of climatological factors on the presence of fungi and mycotoxins in brewers grain was determined by univariate analysis of variance, followed by the Student t-test for the analysis of differences between means of the groups, using the SPSS statistical software. In addition, the correlation between the presence of fungi and mycotoxins was determined using Pearson’s correlation coeficient.
3. Results and discussion
Analysis of the information obtained from the questionnaires applied to the farms studied revealed a semi-intensive animal rearing system with a closely similar feeding regimen consisting of bulky feed and brewers grain, although other agroindustrial residues such as sugarcane were also added to the diet, when available. Brewers grain were oVered at a proportion of 15–20 kg/animal/day to lactating cows; however, in two farms, all cattle received the product. Brewers grain were stored in cement tanks with a capacity of approximately 20 tons in all properties, except for farm C, where this material was kept on a cemented surface covered with a black plastic sheet. The storage period varied as a function of the number of lactating cows, generally ranging from 8 to 15 days. As a conservation measure, common salt was added to the brewers grain as it arrived in the farms.
Analysis of the climatic factors demonstrated no significant difference in minimum or mean temperature between properties, while the maximum temperature in farm E was lower than in the other farms (Table 1). The highest temperatures were observed during the 4th collection period (Table 2).
Rainfall levels and relative humidity were signiWcantly higher during the 1st and 2nd collection periods. The moisture content of the samples also showed significant variation, with the lowest value being observed in farm C, possibly due to the form of storage of brewers grain in this property (Table 1).
Analysis of mycoflora of the 80 brewers grain samples revealed Aspergillus spp. as the most frequent (42.5%) genus isolated, followed by Mucor spp. (32.5%), Rhizopus spp. (32.5%), Penicillium spp. (7.5%), and Fusarium spp. (2.5%). Cladosporium spp. was detected in only one sample.
The predominance of fungi of the genus Aspergillus, together with the reduced presence of Fusarium observed for all samples studied (Table 2) may be the result of brewers grain storage and processing conditions. These conditions may have favored the development of storage and contaminant fungi instead of those known as Weld fungi, which include the genus Fusarium, more frequently found on recently harvested grains than on processed and stored ones (Lillehoj, 1973). Abramson, Hulasare, White, Jayas, and Marquardt (1999) and Hill and Lacey (1983) also found a higher frequency of Aspergillus and Penicillium when analyzing stored barley grains.
Table 1 - Mean climatic conditions in the properties and during each sample collection
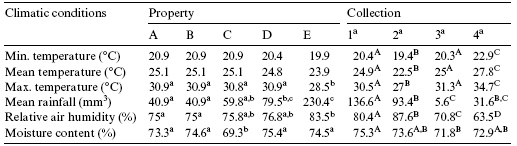
Note: Uppercase shows the comparison of values in the rows between properties, and lowercase shows the comparison of values in the collection of samples (p < 0.05).
Table 2 - Mean fungal contamination in the properties and during each sample collection (£103 CFU/g)
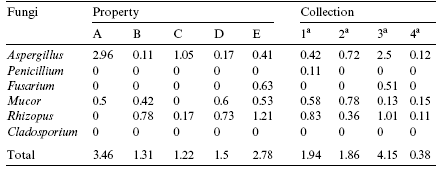
Note: Uppercase shows the comparison of values in the rows between properties, and lowercase shows the comparison of values in the collection of samples (p < 0.05).
Ackermann (1998) and Lee et al. (1986), who analyzed malted barley grains, observed the presence of the genera Alternaria, Aspergillus, Fusarium, Mucor, Penicillium, Cladosporium, and Rhizopus. However, the most frequent fungi found on barley grains collected in the Weld were Alternaria, Fusarium and Cladosporium; Aspergillus and Penicillium were also detected, but in smaller amounts (Ackermann, 1998; Sanchis, Sanclemente, Usall, & Viñas, 1993).
The greatest frequency of fungi was observed during the 3rd collection period (Table 2). The temperature and moisture content of the samples may have influenced these results since the lowest rainfall was observed during this period (Table 1). According to Hill and Lacey (1983), moisture content greater than 40% shows water activity equal to 1, an index favorable to fungal development. The high moisture content of the samples analyzed is the result of conservation of brewers grain in water. Rainfall played no role in this measurement, although this factor usually interferes with air humidity and consequently with the moisture content of the sample. Samples from farm C showed the lowest level of fungal contamination (Table 2), possibly because of the management of brewers grain in this property.
The product was consumed within a maximum period of 5 days and stored in such a way that the existing water could be drained, enabling the drying of the barley.
High moisture content of substrates stored for long periods provides adequate microclimate for fungal development and, consequently, mycotoxin production (Sundlof & Strickland, 1986). Fungi of the genus Fusarium generally invade and colonize grains with moisture content between 22% and 25%, while Aspergillus and Penicillium are found in grains with moisture content between 13% and 18% (Lillehoj, 1973).
Aspergillus spp. were most frequently detected in farm A during the 3rd collection, characterized by mean temperature of 25 °C and 70–75% relative humidity. Fungi of the genus Penicillium were observed during the 1st collection period, when climatic conditions were ideal for fungal proliferation.
The genus Fusarium was detected during the 3rd collection period, when temperature and humidity conditions were adequate despite a low rainfall (Table 2).
The genus Cladosporium was only detected in one sample (0.15x103CFU/g) obtained during the 1st collection in farm E. The genera Mucor and Rhizopus were most frequently found in samples derived from the 2nd and 3rd collections, respectively.
In the present study, no correlation was observed between climatic factors and the frequency of Aspergillus and Penicillium, although Silva, Pozzi, Mallozzi, Ortega, and Correa (2000) reported some of these climatic factors, such as maximum and mean temperature, rainfall and grain water content, to be determining factors in the development of these genera.
A positive correlation was observed between the genus Fusarium, temperature (mean, minimum and maximum) and air humidity. Such correlation was also observed by Pozzi et al. (1995) who investigated the presence of fungi in corn grains. Other factors, such as rainfall and relative humidity, may also inXuence the development of this fungus (Silva et al., 2000). Mean rainfall also correlated with the development of Mucor, Rhizopus and Cladosporium in samples analyzed in the present study.
Analysis of mycotoxins in brewers grain revealed contamination with aXatoxins, while no ochratoxins were detected. Twenty-seven (33.75%) of the 80 samples analyzed were positive for aflatoxins (B1+B2+G1+G2), with levels ranging from 1 to 3 µg/kg (Tables 3 and 4).
Table 3 - Number of samples positive for the presence of aXatoxins (B1 + B2 + G1 + G2) collected from March 2002 to May 2003
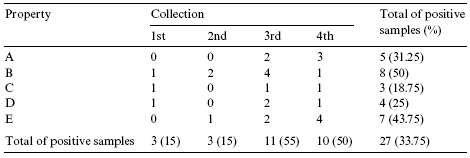
Table 4 - Mean concentration of total aXatoxins (µg/kg) in barley samples collected from March 2002 to May 2003
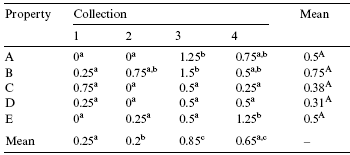
Note: Uppercase shows the comparison of values in the rows, and lowercase shows the comparison of values in the columns (p < 0.05).
The highest aflatoxin concentrations were observed in samples derived from farm B and the lowest levels were detected in farm D, although the diVerence was not signiWcant. As for the collection period, the highest levels were detected in brewers grain samples obtained during the 3rd period (Table 4).
No correlation could be established between the presence of fungi and toxin levels in the samples. However, a positive correlation was observed between aflatoxin level, mean rainfall and relative humidity, factors that have an expressive influence on moisture content of the samples. The lack of a correlation between aflatoxin levels and fungal development/climatic conditions observed in this study suggests that the production of these toxins may have occurred before storage of the brewers grain in the farms, or that the Aspergillus strains detected are able to produce large amounts of toxins.
Scientific reports on the contamination of animal feed with mycotoxins in the State of Bahia are scarce. Only Batatinha et al. (2003) and Bautista et al. (1989) reported the presence of aflatoxins in corn and peanuts, and their byproducts, with an incidence of 12% and 58%, respectively.
In the present study, aXatoxin concentrations (B1+B2+ G1+G2) found in brewers grain were below the levels allowed for animal feed by legal regulations (Decreto MA/ SNAD/SFA no. 7 from November 9, 1988), which establish a maximum level of 50µg/kg for any raw material to be used directly or as an ingredient in feed destined for animal consumption. However, the presence of this mycotoxin in this substrate indicates the existence of contamination, a fact that would require periodic monitoring because of the favorable climatic conditions for fungal development and the inadequate form of storage that favors the proliferation of Aspergillus Xavus and Aspergillus parasiticus, the main producers of these toxins. This is the way by which the occurrence of aflatoxicosis in production animals could be prevented, minimizing economic losses and reducing the risk posed by the transmission of highly carcinogenic metabolites (aflatoxin M1) into milk, both for human and animal health.
Acknowledgements
We would like to thank Fundação de Apoio à Pesquisa do Estado da Bahia (FAPESB), Secretaria de Agricultura do Estado da Bahia (SEAGRI) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the Wnancial support for this study.
References
Abramson, D., Hulasare, R., White, N. D. G., Jayas, D. S., & Marquardt, R. R. (1999). Mycotoxin formation in hulless barley during granary storage at 15% and 19% moisture content. Journal of Stored Products Research, 35, 297–305.
Ackermann, A. (1998). MycoXora of South African barley and malt. Journal of American Society of Brewing Chemists, 56, 169–176.
Aflatest Manual (2002). Vicam protocols.
Anuário estatístico—Bahia—2002 (2002). SEI—Informações a Serviço da Sociedade (Vol. 13. pp. 1–570). Salvador, Bahia.
Batatinha, M. J. M., Santos, M. M., Botura, M. B., Almeida, G. N., Domingues, L. F., Kowaski, C. H., et al. (2003). Ocorrência de aflatoxinas em amendoim e seus produtos comercializados no Estado da Bahia durante o ano de 2002. Revista do Instituto Adolfo Lutz, 62, 183–187.
Bautista, A. R. P. L., Oliveira, M. Z. A., Miranda, M. S., & Sales, L. A. (1989). Aflatoxinas em grãos de milho armazenado no Estado da Bahia. Revista da Sociedade Brasileira de Toxicologia, 2, 24–25.
Council of Agricultural Science and Technology (CAST). (2003). Mycotoxins: risks in plant, animal and human systems. Task Force Report no. 139. EUA: CAST.
Fink-Gremmels, J. (1999). Mycotoxins: their implications for human and animal health. Veterinary Quarterly, 21, 115–120.
Hill, R. A., & Lacey, J. (1983). Factors determining the microflora of stored barley grain. Annals of Applied Biology, 102, 467–483.
Instituto Brasileiro de Geografia e Estatística (IBGE). (2003). Produção da Pecuária Municipal (2002) (Vol. 30). Rio de Janeiro: IBGE. Kim, J. C., Kang, H. G., Lee, D. H., Lee, Y. W., & Yoshizawa, T. (1993).
Natural occurrence of Fusarium mycotoxins (Trichotecenes and zearalenone) in barley and corn in Korea. Applied and Environment Microbiology, 59, 3789–3802.
Lee, U. S., Jang, H. S., Tanaka, T., Toyasaki, N., Sugiura, Y., & Oh, Y. J. (1986). Mycological survey of Korean cereals and production of mycotoxins by Fusarium isolates. Applied and Environment Microbiology, 52, 1258–1260.
Lillehoj, E. B. (1973). Feed sources and conditions conducive to production of aflatoxin, ochratoxin, Fusarium toxins and zearalenone. JAVMA, 163, 1281–1284.
Mallmann, C. A. et al. (2000). Automation of the analytical procedure for the simultaneous determination of aXatoxins AFB1 AFB2, AFG1 and AFG2. In X International IUPAC symposium on mycotoxins and phycotoxins 2000. Guarujá, São paulo, Brasil, 21–25 May (p. 35).
Mallmann, C. A., Santurio, J. M., & Dilkin, P. (1999). Equine leukoencephalomalacia associated with ingestion of corn contaminated with fumonisin B1. Revista de Microbiologia, 30, 249–252.
Nelson, P. E., Tousoun, T. A., & Marasas, W. F. O. (1983). Fusarium species: Na illustrated manual for identiWcation. London: Pennsylvania State University Press.
Ochratest Manual (2002). Vicam protocols. Pozzi, C. R., Correa, B., Gambale, W., Paula, C. R., Chacon-Reche, N. O.,& Meireles, M. C. A. (1995). Post-harvest and stored corn in Brazil: mycoflora interaction, abiotic factors and mycotoxin occurrence. Food Additives and Contaminants, 12(3), 313–319.
Raper, K. B., & Fennell, K. I. (1965). The genus Aspergillus. Baltimore, MD: Baltimore, Willians and Wilkins.
Rodriguez-Amaya, D. B., & Sabino, M. (2002). Mycotoxin research in Brazil: The last decade in review. Brazilian Journal of Microbiology, 33, 1–11.
Sanchis, V., Sanclemente, A., Usall, J., & Viñas, I. (1993). Incidence of mycotoxigenic Alternaria alternata and Aspergillus Xavus in barley. Journal of Food Protection, 56, 246–248.
Silva, D. J. (1991). Análise de alimentos (métodos químicos e biológicos) (pp. 1–11). Viçosa: Impr. University.
Silva, J. B., Pozzi, C. R., Mallozzi, M. A. B., Ortega, E. M., & Correa, B. (2000). MycoXora and occurrence of Aflatoxin B1 and Fumonisin B1 during storage of Brazilian sorghum. Journal of Agricultural and Food Chemistry, 48, 4352–4356.
Sundlof, S. F., & Strickland, C. (1986). Zearalenone and zeranol: potential residue problems in livestock. Veterinary and Human Toxicology, 28, 242–250.
Swanson, K. M. J., Busta, F. F., Peterson, E. H., & Johnson, M. E. (1992). Colony count-methods. In C. Vanderzant & D. S. Splittstoesser (Eds.), Compendium of methods for the microbiological examination of food. New York: American Public Health Association.
Authors: Monica M.S. Simas a, Mariana B. Botura a, Benedito Correa b, Myrna Sabino c, Carlos A. Mallmann d, Thereza C.B.S.C. Bitencourt a, Maria J.M. Batatinha a
a Escola de Medicina Veterinária, Universidade Federal da Bahia, Av. Adhemar de Barros, 500, CEP 40210-170 Salvador, BA, Brazil
b Departamento de Microbiologia, Instituto de Ciências Biomédicas, Universidade de São Paulo, Av. Prof. Lineu Prestes, 1374, CEP 05508-900 São Paulo, SP, Brazil
c Instituto Adolfo Lutz, Av. Dr. Arnaldo, 355, CEP 01246-902 São Paulo, SP, Brazil d Universidade Federal de Santa Maria, Faixa de Camobi, Km 9, Campus Universitário, CEP 97105-900 Santa Maria, RS, Brazil
Related topics:
Recommend
Comment
Share

27 de marzo de 2008
Very informative.
Thanx.
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.





.jpg&w=3840&q=75)