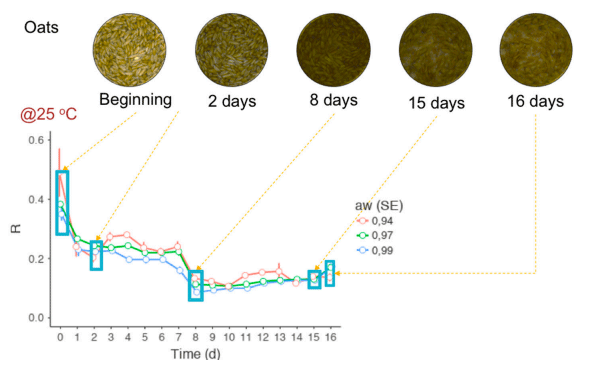
Why RGB Imaging Should be Used to Analyze Fusarium Graminearum Growth and Estimate Deoxynivalenol Contamination
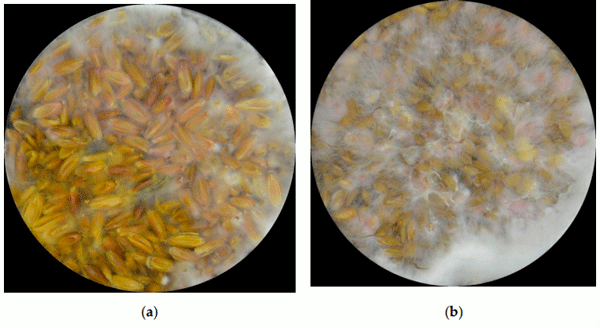
Size-based fungal growth studies are limited because they do not provide information about the mold’s state of maturity, and measurements such as radius and diameter are not practical if the fungus grows irregularly. Furthermore, the current methods used to detect diseases such as Fusarium head blight (FHB) or mycotoxin contamination are labor-intensive and time consuming. FHB is frequently detected through visual examination and the results can be subjective, depending on the skills and experience of the analyzer. For toxin determination (e.g., deoxynivalenol (DON), the best methods are expensive, not practical for routine. RGB (red, green and blue) imaging analysis is a viable alternative that is inexpensive, easy to use and seemingly better if enhanced with statistical methods. This short communication explains why RGB imaging analysis should be used instead of size-based variables as a tool to measure growth of Fusarium graminearum and DON concentration.
Keywords: RGB; Fusarium graminearum; growth; deoxynivalenol.
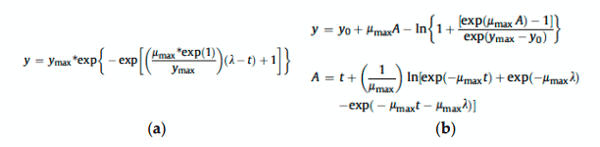
1. Saccon, F.A.; Parcey, D.; Paliwal, J.; Sherif, S.S. Assessment of Fusarium and deoxynivalenol using optical methods. Food Bioprocess Technol. 2017, 10, 34–50. [CrossRef]
2. Velluti, A.; Sanchis, V.; Ramos, A.J.; Turon, C.; Marin, S. Impact of essential oils on growth rate, zearalenone and deoxynivalenol production by Fusarium graminearum under different temperature and water activity conditions in maize grain. J. Appl. Microbiol. 2004, 96, 716–724. [CrossRef] [PubMed]
3. Ryu, D.; Bullerman, L.B. Effect of cycling temperatures on the production of deoxynivalenol and zearalenone by Fusarium graminearum NRRL 5883. J. Food Prot. 1999, 62, 1451–1455. [CrossRef] [PubMed]
4. Fredlund, E.; Gidlund, A.; Sulyok, M.; Borjesson, T.; Krska, R.; Olsen, M.; Lindblad, M. Deoxynivalenol and other selected Fusarium toxins in Swedish oats—Occurrence and correlation to specific Fusarium species. Int. J. Food Microbiol. 2013, 167, 276–283. [CrossRef] [PubMed]
5. Leplat, J.; Friberg, H.; Abid, M.; Steinberg, C. Survival of Fusarium graminearum, the causal agent of Fusarium head blight. A review. Agron. Sustain. Dev. 2013, 33, 97–111. [CrossRef]
6. Jaillais, B.; Roumet, P.; Pinson-Gadais, L.; Bertrand, D. Detection of Fusarium head blight contamination in wheat kernels by multivariate imaging. Food Control 2015, 54, 250–258. [CrossRef]
7. Langseth, W.; Elen, O. Differences between barley, oats and wheat in the occurrence of deoxynivalenol and other trichothecenes in Norwegian grain. J. Phytopathol. 1996, 144, 113–118. [CrossRef]
8. Cambaza, E.M.; Koseki, S.; Kawamura, S. Meta-analytic review on the impact of temperature and water activity in deoxynivalenol synthesis by Fusarium graminearum. Food Res. 2018, 2, 443–446. [CrossRef]
9. Dvoˇráˇcek, V.; Prohasková, A.; Chrpová, J.; Štoˇcková, L. Near infrared spectroscopy for deoxynivalenol content estimation in intact wheat grain. Plant Soil Environ. 2012, 58, 196–203. [CrossRef]
10. Sabater-Vilar, M.; Malekinejad, H.; Selman, M.H.; van der Doelen, M.A.; Fink-Gremmels, J. In vitro assessment of adsorbents aiming to prevent deoxynivalenol and zearalenone mycotoxicoses. Mycopathologia 2007, 163, 81–90. [CrossRef]
11. Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [CrossRef] [PubMed]
12. Shahin, M.; Hatcher, D.; Symons, S. Development of multispectral imaging systems for quality evaluation of cereal grains and grain products. In Computer Vision Technology in the Food and Beverage Industries; Elsevier: Amsterdam, The Netherlands, 2012; pp. 451–482.
13. Wiwart, M.; Koczowska, I.; Borusiewicz, A. Estimation of Fusarium head blight of triticale using digital image analysis of grain. In Proceedings of International Conference on Computer Analysis of Images and Patterns; Springer: Berlin/Heidelberg, Germany, 2011; pp. 563–569.
14. Cavret, S.; Laurent, N.; Videmann, B.; Mazallon, M.; Lecoeur, S. Assessment of deoxynivalenol (DON) adsorbents and characterisation of their efficacy using complementary in vitro tests. Food Addit. Contam. 2010, 27, 43–53. [CrossRef] [PubMed]
15. Martins, M.L.; Martins, H.M. Influence of water activity, temperature and incubation time on the simultaneous production of deoxynivalenol and zearalenone in corn (Zea mays) by Fusarium graminearum. Food Chem. 2002, 79, 315–318. [CrossRef]
16. Greenhalgh, R.; Neish, G.A.; Miller, J.D. Deoxynivalenol, acetyl deoxynivalenol, and zearalenone formation by Canadian isolates of Fusarium graminearum on solid substrates. Appl. Environ. Microbiol. 1983, 46, 625–629. [PubMed]
17. He, J.; Yang, R.; Zhou, T.; Tsao, R.; Young, J.C.; Zhu, H.; Li, X.Z.; Boland, G.J. Purification of deoxynivalenol from Fusarium graminearum rice culture and mouldy corn by high-speed counter-current chromatography. J. Chromatogr. A 2007, 1151, 187–192. [CrossRef] [PubMed]
18. Morooka, N.; Uratsuji, N.; Yoshisawa, T.; Yamamoto, H. Studies on the toxic substances in barley infected with Fusarium spp. Food Hyg. Saf. Sci. 1972, 13, 368–375. [CrossRef]
19. Dammer, K.-H.; Möller, B.; Rodemann, B.; Heppner, D. Detection of head blight (Fusarium ssp.) in winter wheat by color and multispectral image analyses. Crop Prot. 2011, 30, 420–428. [CrossRef]
20. Garcia, D.; Barros, G.; Chulze, S.; Ramos, A.J.; Sanchis, V.; Marin, S. Impact of cycling temperatures on Fusarium verticillioides and Fusarium graminearum growth and mycotoxins production in soybean. J. Sci. Food Agric. 2012, 92, 2952–2959. [CrossRef] [PubMed]
21. Almeida, M.I.; Almeida, N.G.; Carvalho, K.L.; Goncalves, G.A.; Silva, C.N.; Santos, E.A.; Garcia, J.C.; Vargas, E.A. Co-occurrence of aflatoxins B(1), B(2), G(1) and G(2), ochratoxin A, zearalenone, deoxynivalenol, and citreoviridin in rice in Brazil. Food Addit. Contam. 2012, 29, 694–703. [CrossRef] [PubMed]
22. Bergsjo, B.; Langseth, W.; Nafstad, I.; Jansen, J.H.; Larsen, H.J. The effects of naturally deoxynivalenol-contaminated oats on the clinical condition, blood parameters, performance and carcass composition of growing pigs. Vet. Res. Commun. 1993, 17, 283–294. [CrossRef]
23. Yoshizawa, T. Thirty-five Years of Research on Deoxynivalenol, a Trichothecene Mycotoxin: With Special Reference to Its Discovery and Co-occurrence with Nivalenol in Japan. Food Saf. 2013, 1, 2013002. [CrossRef]
24. Langseth, W.; Stabbetorp, H. The effect of lodging and time of harvest on deoxynivalenol contamination in barley and oats. J. Phytopathol. 1996, 144, 241–245. [CrossRef]
25. Jirsa, O.; Polišenská, I. Identification of Fusarium damaged wheat kernels using image analysis. Acta Univ. Agric. Silvic. Mendel. Brun. 2014, 59, 125–130. [CrossRef]
26. Cambaza, E.; Koseki, S.; Kawamura, S. The Use of Colors as an Alternative to Size in Fusarium graminearum Growth Studies. Foods 2018, 7, 100. [CrossRef] [PubMed]
27. Benaliaa, S.; Bernardi, B.; Cuberob, S.; Leuzzic, A.; Larizzac, M.; Blascob, J. Preliminary trials on hyperspectral imaging implementation to detect mycotoxins in dried figs. Chem. Eng. 2015, 44, 157–162.
28. Garcia, D.; Ramos, A.J.; Sanchis, V.; Marin, S. Predicting mycotoxins in foods: A review. Food Microbiol. 2009, 26, 757–769. [CrossRef] [PubMed]
29. Gupta, S.D.; Ibaraki, Y.; Trivedi, P. Applications of RGB color imaging in plants. Plant Image Anal. Fundam. Appl. 2014, 41, 22.
30. Padmavathi, K.; Thangadurai, K. Implementation of RGB and grayscale images in plant leaves disease detection–comparative study. Indian J. Sci. Technol. 2016, 9, 1–6. [CrossRef]
31. Teena, M.; Manickavasagan, A.; Al-Sadi, A.M.; Al-Yahyai, R.; Deadman, M. RGB color imaging to detect Aspergillus flavus infection in dates. Emir. J. Food Agric. 2016, 28, 683–688. [CrossRef]
32. Yoon, S.-C.; Shin, T.-S.; Heitschmidt, G.W.; Lawrence, K.C.; Park, B.; Gamble, G. Hyperspectral imaging using a color camera and its application for pathogen detection. In Proceedings of the Image Processing: Machine Vision Applications VIII, San Francisco, CA, USA, 13 March 2015; p. 940506.
33. Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42.
34. Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [CrossRef] [PubMed]
35. Burger, W.; Burge, M.J. Digital Image Processing: An Algorithmic Introduction Using Java; Springer: Berlin, Germany, 2016.
36. Sugiura, Y. Gibberella zeae (Schwabe) Petch. In JCM Catalogue; Japan Collection of Microorganisms, Ed.; Microbe Division (JCM): Tsukuba, Japan, 1996.
37. Sugiura, Y.; Watanabe, Y.; Tanaka, T.; Yamamoto, S.; Ueno, Y. Occurrence of Gibberella zeae strains that produce both nivalenol and deoxynivalenol. Appl. Environ. Microbiol. 1990, 56, 3047–3051. [PubMed]
38. Cambaza, E. Comprehensive Description of Fusarium graminearum Pigments and Related Compounds. Foods 2018, 7, 165. [CrossRef] [PubMed]
39. Cambaza, E.; Koseki, S.; Kawamura, S. Fusarium graminearum Colors and Deoxynivalenol Synthesis at Different Water Activity. Foods 2018, 8, 7. [CrossRef] [PubMed]
40. Cambaza, E.; Koseki, S.; Kawamura, S. Fusarium graminearum growth and its fitness to the commonly used models. Int. J. Agric. Environ. Food Sci. 2019, 3, 10–14. [CrossRef]
41. Cambaza, E.; Koseki, S.; Kawamura, S. Fusarium graminearum colors and deoxynivalenol synthesis at different temperatures. In Proceedings of the 2nd International Conference on Food Quality, Safety and Security (FOOD QualSS), Colombo, Sri Lanka, 25–26 October 2018; p. 15.
42. Cambaza, E.; Koseki, S.; Kawamura, S. Comprehensive description of Fusarium graminearum pigments. In Proceedings of the 8th Academic Exchange for Collaborative Research Between ETHZ and Hokkaido University (AECoR8), Sapporo, Hokkaido, 7–9 November 2018.
43. Cambaza, E.; Shigenobu, K.; Kawamura, S. RGB Imaging as Tool to Analyze the Growth of Fusarium graminearum in Infected Oats (Avena sativa) and Rice (Oryza sativa). In Preprints; MDPI: Basel, Switzerland, 2019. [CrossRef]
44. Olivares Diaz, E. Physical and Chemical Properties of Multiple Varieties of NERICA, Indica and Japonica Types of Rice for Assessing and Enhancing Quality; Hokkaido University: Sapporo, Hokkaido, Japan, 2018.
45. Ruan, R.; Ning, S.; Song, A.; Ning, A.; Jones, R.; Chen, P. Estimation of Fusarium scab in wheat using machine vision and a neural network. Cereal Chem. 1998, 75, 455–459. [CrossRef]
46. Taylor, D.R.; Aarssen, L.W.; Loehle, C. On the relationship between r/K selection and environmental carrying capacity: A new habitat templet for plant life history strategies. Oikos 1990, 58, 239–250. [CrossRef]
47. Choi, S.; Jun, H.; Bang, J.; Chung, S.H.; Kim, Y.; Kim, B.S.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Behaviour of Aspergillus flavus and Fusarium graminearum on rice as affected by degree of milling, temperature, and relative humidity during storage. Food Microbiol. 2015, 46, 307–313. [CrossRef] [PubMed]





