Recognizing the reality of the aquaculture mycotoxin problem: searching for a common and effective solution
In a previous paper (Spring and Fegan, 2005), we presented information reviewing current knowledge of mycotoxins as they relate to aquaculture species. Since then, awareness of mycotoxin-related issues within the industry has grown as feed manufacturers and producers realize the importance of mycotoxins other than aflatoxin and their potential to impact production as well as the prevalence of mycotoxins in many raw materials. While it remains true that the majority of mycotoxin issues are the result of poor on-farm storage conditions, there is likely to be an increased risk of exposure to mycotoxins in raw materials used for aquafeeds, either farm-made or formulated.
The increasing cost of raw materials for aquaculture feeds has sparked an increased use of plant proteins as substitutes for fish meal. More recently, the cost of plant-based raw materials has itself begun to rise, partly due to the increased demand for biofuels. As a consequence, the volume, and quality, of affordable plant materials available for animal feeds has fallen. Feed manufacturers are now faced with either increasing the price of aquaculture feeds or using raw materials that may increase their risk of contamination with one or more mycotoxins.
Mycotoxin contamination has also been thought of as a strictly seasonal issue in many countries. However, the increasing globalization of trade and incorporation of imported raw materials in aquafeeds exposes feed manufacturers and their clients to the risk of combinations of mycotoxins either from multiple mycotoxins in the same raw material or from different mycotoxins in different ingredients in the same formulation.
Mycotoxin contamination in raw materials and finished feeds
To determine the extent of the possible risk, a series of surveys of raw materials and finished aquafeeds was undertaken in several Asian countries (Tables 1 and 2). The surveys clearly showed that levels of mycotoxins in some raw materials exceeded the levels regarded as suitable for feed materials and that most of the contaminated raw materials contained several mycotoxins.
Table 1. Mycotoxin contamination of selected raw materials in Asia.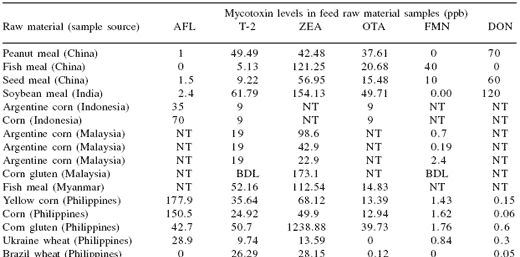
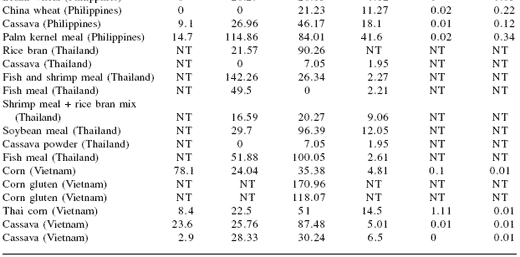
NT = not tested; BDL = below detection limit
AFL: aflatoxin, ZEA: zearalenone, OTA: ochratoxin, FMN: fumonisin, DON: deoxynivalenol (vomitoxin)
Most feed companies include testing for aflatoxin contamination as a part of their Hazard Analysis and Critical Control Point (HACCP) process. Relatively few also include testing for other mycotoxins on a routine basis on the assumption that, if aflatoxin is absent, there is little risk of other mycotoxins being present. The survey data clearly show this assumption to be false since several of the raw materials tested that had negligible levels of aflatoxin contamination had significant levels of other mycotoxins such as ochratoxin, T-2 or zearalenone.
The results for T-2 and zearalenone in fish meal are confusing since these are Fusarium mycotoxins. Fusarium molds are generally found in field conditions rather than storage so their appearance in fish meal is possibly an anomaly due to the use of ELISA tests. In our investigation of this issue, we found that previous studies (Bauer, unpublished) indicated that there may be some interaction between fish meal and ELISA tests for T- 2 and zearalenone and that HPLC would be the preferred method of analysis.
Table 2. Mycotoxin levels found in finished aquaculture feeds in several Asian countries.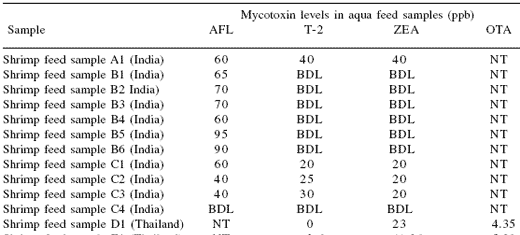
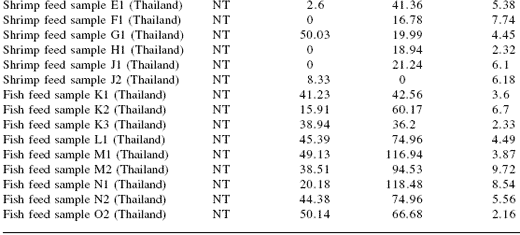
NT = not tested; BDL = below detection limit
AFL: aflatoxin, ZEA: zearalenone, OCT: ochratoxin
Some strains of Fusarium including F. oxysporum and F. solani are known to be opportunistic pathogens of fish and shrimp (Hatai et al., 1986; Lightner, 1996; Ostland et al., 1987; Souheil et al., 1999). None of these studies specifically investigated the role of Fusarium toxins in these infections, but Souheil et al. (1999) found that injection of crude filtrates of F. oxysporum into kuruma prawn (Penaeus japonicus) caused some of the same symptoms noted in animals infected with whole fungus.
The levels of mycotoxins found in the finished feeds indicate that mycotoxins in raw materials can result in feeds with mycotoxin contamination that, in the case of aflatoxins, can exceed the limits set for animal feeds in some countries (Table 3) (FAO, 2004). Although the levels were generally quite low, the effects of multiple mycotoxin exposure have been little studied in aquatic species. Many of the previous studies have focused on determining the acute toxicity of single, purified mycotoxins. However, based on the survey data, a more realistic scenario would be chronic exposure to lower levels of multiple mycotoxins.
Multiple mycotoxins
Multiple mycotoxin exposure has been demonstrated to have synergistic, antagonistic or additive effects in terrestrial species such as poultry or swine (Wyatt, 2005). Research into the combined effects of two or more mycotoxins is much less common in aquatic species than research into the exposure of single mycotoxins.
However, where such research has been conducted, it also points to significant interactive effects. Yildirim et al. (2000), for example, investigated the toxicity of moniliformin (MON) and fumonisin B1 (FB1), singly and in combination, to young channel catfish (Ictalurus punctatus) and found some interaction between MON and FB1 in feed. A combination of MON and FB1 at 40:40 mg/kg gave a significant decrease in blood cell count and liver cell size and an increase in serum pyruvate. Although FB1 significantly increased the free sphinganine:free sphingosine ratio, there was no additional effect of adding MON.
More importantly from a production standpoint, weight gain was significantly affected by the two mycotoxins in combination. The weight gain of fish exposed to the mycotoxin combination was 42% lower than the control compared with feeding MON or FB1 singly at the same level (16% and 23% reduction, respectively). They concluded that the combination of these two mycotoxins could have an enhanced detrimental effect on growth rate and health of channel catfish, especially since they can both be produced by some species of Fusarium and are known to be found in the same raw materials.
Table 3. Mycotoxin limits in animal feeds from selected countries (FAO, 2004).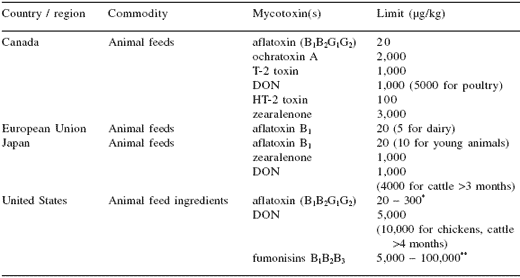
*20: immature animals; 100: breeding swine, cattle, all mature poultry; 200: finishing swine >100 lb; 300: beef cattle, swine, poultry
**5,000: equine, rabbits; 20,000: swine, catfish; 30,000: breeders, layers; 60,000: ruminants; 100,000 poultry
Several studies into the role of mycotoxins as potential carcinogens have used fish species as models, especially rainbow trout (Oncorhynchus mykiss) due to its sensitivity to aflatoxin-induced tumours. Some studies have specifically investigated the interactions between mycotoxins in tumour development. Carlson et al. (2001) found that FB1 promoted aflatoxin-initiated liver tumors in fish fed more than 23 ppm for 42 weeks, possibly due to disruption of sphingolipid metabolism.
Similarly, McKean et al. (2006) studied the combined effects of aflatoxin B1 and T-2 toxin in rats, mosquitofish (Gambusia affinis), immortalized human hepatoma cells and human bronchial epithelial cells. Preliminary experiments to establish acute toxicity to mosquitofish for the individual toxins showed that the median lethal dose (LD50) for aflatoxin B1 was 681 μg/L and the LD50 for T-2 was 147 μg/L. The authors then tested the combination of aflatoxin and T-2 at different dilutions based on the LD50 (Table 4).
They then determined the combined effect of the mycotoxins by comparing the estimated LD50 of the mycotoxin combination against the theoretical value assuming no significant interaction. A significant additive interaction in the toxic response to the combination of mycotoxins was observed over the 5-day test period with the measured LD50 (combined) being lower (234.26 μg/L) than the theoretical LD50 (414 μg/L).
Table 4. Combined toxicity of aflatoxin B1 and T-2 toxin in mosquitofish.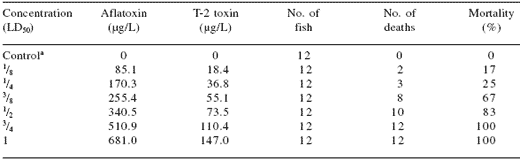
aControl animals received DMSO
From McKean et al., 2006
The impact of other compounds on mycotoxin toxicity is also becoming apparent. In terrestrial animals, a link between the extent of mycotoxicoses and nutrients in the diet has long been recognized (Wyatt, 2005). Some of the specific interactions have been reviewed by Schaeffer and Hamilton (1991) including interactions between vitamins, protein, fat and specific amino acids such as methionine. Together with mycotoxin-induced damage to the intestine and the impact of mycotoxins on nutrient uptake, these interactions can greatly amplify the impact of mycotoxins in a production situation.
In one study (Oganesian et al., 1999), rainbow trout (Oncorhynchus mykiss) were found to be more susceptible to aflatoxin B1-induced hepatocarcinogenesis when given feeds containing indole-3-carbinol (I3C), a plant metabolite found in broccoli, cauliflower and brussels sprouts. Tumor incidence in fish exposed to aflatoxin increased with increasing I3C in the diet (Figure 1).
These studies indicate that mycotoxin, and particularly aflatoxin, contamination of feeds may be a widespread phenomenon. The possibility of mycotoxins from other sources in aquaculture production has not been studied. In pond culture, for example, fish and shrimp can derive a significant proportion of their nutrition from natural food items in the pond itself. In more extensive culture systems, food from the aquatic environment is the only source of nutrition available to the animal. There has been little research on mycotoxins in the aquatic environment although, as previously mentioned, Fusarium can be an opportunistic pathogen of fish and shrimp.
Feeds may also be exposed to conditions that encourage the production of mycotoxins after leaving the mill. Farmers may store feeds in poor conditions or feeds may become wet during transport and storage, causing them to become contaminated with mycotoxins.
Several studies have shown that improperly stored feeds can have high levels of aflatoxins.
In the Philippines, 59 out of 62 samples of shrimp feed had detectable levels of aflatoxin and 22% of the farm-stored feeds tested had aflatoxin levels exceeding 50 ppb (Bautista et al., 1994). Another study in Mexico (Burgos-Hernández et al., 2003) detected aflatoxins and fumonisins in feed from all areas sampled and aflatoxin and fumonisin in some samples exceeded safe levels. Aflatoxin B1 was also detected in shrimp feed samples in Thailand up to a level of 0.651 ppb (Bintvihok et al., 2003).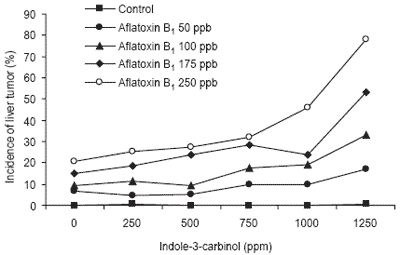
Figure 1. Incidence of liver tumors in rainbow trout exposed to aflatoxin B1 and indole-3-carbinol (after Oganesian et al., 1999).
The environment is an important factor in the success or failure of any aquaculture operation and many production problems can be traced back to stresses caused by suboptimal environmental conditions. Interactions between environmental factors, chemical compounds in the water and soil, and mycotoxins in feeds may represent an important contributing factor to mycotoxin-influenced production problems.
Few studies to date have used naturally contaminated raw materials and, where they have, they have focused on only one specific toxin (Cagauan et al., 2005). Given the apparent ubiquity of mycotoxin contamination of raw materials and the lack of information on chronic simultaneous exposure to multiple mycotoxins, there is a need for more studies on the effects of mycotoxin-contaminated raw materials.
Solutions to mycotoxin issues in feeds
Recognizing the fact that mycotoxin contamination of raw materials is an issue, feed manufacturers can take steps to reduce the impact of mycotoxins through the adoption of an appropriate HACCP strategy that includes mycotoxins as one of the critical control points. This would require an appropriate sampling and analysis strategy for mycotoxins identified as risks in the various raw materials and adoption of a suitable control strategy (FAO, 2001; Whitaker et al., 2005).
Prevention of storage mycotoxins in raw materials and feeds is usually done through a combination of good management practices (GMP) for materials storage and use of mold-inhibiting compounds. Mold inhibitors, generally buffered organic acids such as acetic acid or propionic acid, are used to lower the pH of the feed to prevent mold growth.
Mold inhibitors do not affect the toxicity of any mycotoxins that may have been produced by mold contamination before their use, and many molds can survive the high temperatures used in the animal feed manufacturing process (Hughes et al., 1999; Wolf- Hall et al., 1999; Razzazi-Fazeli et al., 2001). The next line of defense is to prevent or limit the uptake of mycotoxins from the intestine using specialized feed additives, commonly referred to as mycotoxin adsorbents (Huwig et al., 2001; Ramos et al., 1996). Among these are:
• Clays
- Hydrated sodium calcium aluminosilicate (HSCAS)
- Zeolites
- Other clays
• Alfalfa fiber
• Polymeric glucomannan (Mycosorb®)
• Enzyme additives
Clays
Some zeolites and aluminosilicate clays have been shown to bind aflatoxin through the ability of the silicate mineral to sequester positively charged or cationic compounds (Diaz and Smith, 2005). They have, however, been largely ineffective in binding to other mycotoxins (Huff et al., 1992, Kubena et al., 1993; 1998) and the US FDA, in a CVM update (FDA, 1999), stated their position that “The use of sodium aluminosilicate and hydrated calcium sodium aluminosilicate as binders for mycotoxins is not considered to be generally recognized as safe (GRAS).”
The basis for this position is that claims for these clays, normally used as anti-caking agents, as mycotoxin binders are unsubstantiated. Concerns were expressed that different mycotoxins may not be uniformly bound and that there may be subsequent release of bound mycotoxins in the gut.
Another issue that may reduce the effectiveness of clays as mycotoxin sequestrants in aquaculture feeds is salinity. Salt water contains many cations such as sodium, potassium and magnesium. These compete for the binding sites in the clay and this is the major reason for the effectiveness of zeolite as an ammonia binder being restricted to freshwater, as the high relative concentration of these cations at even very low salinities reduces the availability of the binding sites to ammonia.
In fact, zeolite filters used as ion exchange columns in freshwater recirculation systems are usually re-charged by flushing with salt water, replacing the ammonia with sodium. Similarly, the high concentration of cations in salt water may also act to reduce the ability of the clay to bind mycotoxins once the feed is exposed to the water.
Alfalfa fiber
Alfalfa fiber can provide some protection against zearalenone (James and Smith, 1982; Stangroom and Smith, 1984) and T-2 toxin (Carson and Smith, 1983) but may itself be a potential source of Fusarium contamination. It is unsuitable in aquaculture diets due to the high inclusion rates required (15-25% of the feed for pigs, rats) and potential for negative impacts of high fiber in aquaculture diets (Shiau et al., 1989).
Polymeric glucomannan: Mycosorb®
Research conducted by Devegowda and co-workers into mycotoxins in poultry, indicated that the inclusion of yeast in the diet reduced the incidence and severity of mycotoxinrelated pathology (Devegowda et al., 1995; 1996). Subsequent research revealed that this effect was associated with the yeast cell wall and, specifically, the glucan polysaccharide layer.
In development of Mycosorb® (Alltech Inc.), the structure of the glucan layer was altered through various modifications to increase its affinity for mycotoxins. These modifications increased the effective surface area of the glucan layer available to bind to mycotoxins and increased the affinity and rate of binding over the native cell wall product (for a review of the use of modified (1-3)-ß-D-glucans, see Zekovic et al., 2005).
The mechanism of action of Mycosorb® has been extensively studied (Yiannikouris et al., 2003; 2004a,b,c; 2005) to elucidate how it can bind to a wide variety of mycotoxins.
The ß-glucan of the yeast cell wall has a complex 3-dimensional structure of glucan triple-helix chains. It is the single helix conformation of pure ß-glucan that provides the greatest number of potential mycotoxin binding sites. Mycotoxin binding occurs through a number of molecular processes, including hydrogen bonding and stabilizing van der Waals interactions forming a stable bond in which the mycotoxin is trapped inside the ß-glucan structure.
Enzyme preparations
Specific enzyme additives that are claimed to detoxify mycotoxins in the digestive tract are also available in a process known as ‘biotransformation.’ These enzymes, produced by specific strains of bacteria and yeast, are claimed to break down mycotoxins into harmless metabolites.
The difficulty with the enzymatic approach is to identify enzymes that are sufficiently non-specific to detoxify a range of mycotoxins but at the same time specific enough to not interfere with digestive function (Smith, 2005). There are also issues with heat stability of enzymes in pelleted or extruded feeds, especially in aquafeeds where there is an extended post-conditioning period in which the feed is exposed to higher temperatures to increase binding and water stability.
Nutritional supplementation
Some studies have also investigated the use of nutritional supplements to reduce mycotoxicoses in aquatic animals. Shalaby (2005) found that elevated vitamin C levels in feeds for tilapia (Oreochromis niloticus) could alleviate some of the toxic effects of ochratoxin. Addition of vitamin C (500 mg/kg diet) enhanced red blood cell count, hemoglobin levels and hematocrit in the blood, and normalized liver function in fish fed ochratoxin at 400 and 600 μg/kg in the feed.
Sahoo and Mukherjee (2002) also found that boosting α-tocopherol levels in the feed alleviated the immunosuppressive effects of aflatoxin in feeds for carp (Labeo rohita). This alleviation may also be related to improved antioxidant status in the fish since mycotoxins are known to exert some influence on antioxidant status (Surai and Dvorska, 2005).
| Conclusions Determining mycotoxin binding capacity of compounds is rarely simple and in vitro trials do not provide a reliable indicator of the efficacy of a compound under field conditions. Simple in vitro systems, in which single mycotoxins are tested using constant pH and fixed incubation times, do not correlate well with in vivo feeding trials in terrestrial animals (Garcia et al., 2003). It is impossible to exactly duplicate the pH conditions and the residency time in the various sections of the intestinal tract (Rotter et al., 1989; Van der Eijk, 2003) and even the most sophisticated in vitro models (Zeijdner and Havenaar, 2000; Garcia et al., 2003) cannot account for environmental and other confounding factors that can influence the severity of mycotoxicoses under field conditions. Factors such as stocking density, health status, nutritional status, disease challenges and salinity and temperature stresses could exaggerate the immunosuppressive effects of aflatoxin, ochratoxin, fumonisin and trichothecenes and comparisons based solely on in vitro results against single mycotoxins under standard conditions must be interpreted with caution. Trial conditions should mimic the actual conditions under which mycotoxin exposure will take place, particularly pH, temperature and, for aquaculture species, salinity. It is also important to consider the speed of mycotoxin adsorption since mycotoxins can be absorbed from the intestine relatively quickly. Thus, trials comparing adsorption over extended periods of several hours may be meaningless since the mycotoxin will have either been absorbed or excreted long before the end of the trial period. In vivo testing of mycotoxin adsorbents is the best guide to efficacy even though in vivo testing in the laboratory may give only a general guide compared with in vivo testing at the farm. There is a dearth of in vivo trials of mycotoxin adsorbents in aquaculture species, partly due to a low recognition of mycotoxin contamination as an issue in aquacultured species. This situation should be addressed given the increased risk of mycotoxin exposure in feeds and raw materials. |
References
Authors: DANIEL F. FEGAN1 and PETER SPRING2Bautista, M.N, C.R. Lavilla-Pitogo, P.F. Subosa and E.T. Begino. 1994. Aflatoxin B1 contamination of shrimp feeds and its effect on growth and hepatopancreas of pre-adult Penaeus monodon. J. Sci. Food Agri. 65:5-11.
Bintvihok, A., A. Ponpornpisit, J. Tangtrongpiros, W. Panichkriangkrai, R. Rattanapanee, K. Doi and S. Kumagai. 2003. Aflatoxin contamination in shrimp feed and effects of aflatoxin addition to feed on shrimp production. J. Food Protect. 66(5):882-885.
Burgos-Hernández, A., S.L. Farías, B. Morales-Leyva, W. Torres-Arreola and J.M. Ezquerra- Brauer. 2003 Presence of mycotoxins in feed for shrimp and their effect on semi-purified trypsin from the hepatopancreas of white shrimp. 54th Annual Meeting of Pacific Fisheries Technologists, Columbia Maritime Museum, Astoria, OR, USA, Feb 23-26.
Cagauan, A.G., R.H. Tayaban, J.R. Somga and R.M. Bartolome. 2005. Effect of aflatoxincontaminated feeds in Nile tilapia (Oreochromis niloticus L.). In: Proc. 6th Int. Symp. on Tilapia Aquaculture (R. Bolivar, G. Mair and K. Fitzsimmons, eds). Publ. American Tilapia Association, Charlestown, VA, USA, pp 172-178.
Carlson, D.B., D.E. Williams, J.M. Spitsbergen, P.F. Ross, C.W. Bacon, F.I. Meredith and R.T. Riley. 2001. Fumonisin B1 promotes aflatoxin B1 and N-methyl-N’-nitronitrosoguanidine- initiated liver tumors in rainbow trout. Toxicol. Appl. Pharmacol. 172(1):29-36.
Carson, M.S. and T.K. Smith. 1983. Effect of feeding alfalfa and refined plant fibres on the toxicity and metabolism of T-2 toxin in rats. J. Nutr. 113:304.
Devegowda, G., B.I.R. Arvind, M.G. Morton and K. Rajendra. 1995. A biotechnological approach to counteract aflatoxicosis in broiler chickens and ducklings by the use of Saccharomyces cerevisiae. In: Proc. Feed Ingredients Asia ’95, pp. 161-171.
Devegowda, G., B.I.R. Arvind and M.G. Morton. 1996. Saccharomyces cerevisiae and mannan oligosaccharides to counteract aflatoxicosis in broilers. Proc. Australian Poultry Science Symposium 8:103-106.
Diaz, D. and T.K. Smith. 2005. Mycotoxin sequestering agents: practical tools for neutralization of mycotoxins. In: The Mycotoxin Blue Book (D. Diaz, ed). Nottingham University Press, UK, pp. 323-339.
FAO. 2001. Manual on the Application of the HACCP System in Mycotoxin Prevention and Control. FAO Food And Nutrition Paper 73. Food and Agriculture Organisation, Rome, p. 114.
FAO. 2004. Worldwide regulations for mycotoxins in food and feed in 2003. FAO Food and Nutrition Paper 81. Food and Agriculture Organisation, Rome, p. 180. FDA. 1999. CVM position on mycotoxin binding claims on anticaking agents. http:// www.fda.gov./cvm/mycoxtup.html.
García, A.R., E. Avila, R. Rosiles and V.M. Petrone. 2003. Evaluation of two mycotoxin binders to reduce toxicity of broiler diets containing ochratoxin A and T-2 toxin contaminated grain. Avian Dis. 47(3):691-699.
Hatai, K., S.S. Kubota, N. Kida and S.-I. Udagawa. 1986. Fusarium oxysporum in Red Sea Bream (Pagrus spp.). J. Wildlife Dis. 22(4):570-571.
Huff, W.E., L.F. Kubena, R.B. Harvey and T.D. Phillips. 1992. Efficacy of hydrated sodium calcium aluminosilicate to reduce the individual and combined toxicity of aflatoxin and ochratoxin A. Poult. Sci. 71:64-69.
Hughes, D.M., M.J. Gahl, C.H. Graham and S.L. Grieb. 1999. Overt signs of toxicity to dogs and cats of dietary deoxynivalenol. J. Anim. Sci. 77:693-700.
Huwig, A., S. Freimund, O. Käppeli and H. Dutler. 2001. Mycotoxin detoxification of animal feed by different adsorbents. Tox. Lett. 122:179-188.
James, L.J. and T.K. Smith. 1982. Effect of dietary alfalfa on zearalenone toxicity and metabolism in rats and swine. J. Anim. Sci. 55(1):110-8.
Kubena, L.F., R.B. Harvey, R.H. Bailey, S.A. Buckley and G.E. Rottinghaus. 1998. Effects of a hydrated sodium calcium aluminosilicate (T-Bind™) on mycotoxicosis in young broiler chickens. Poult. Sci. 77:1502-1509.
Kubena, L.F., R.B. Harvey, W.E. Huff, M.H. Elissalde, A.G. Yersin, T.D. Phillips and G.E. Rottinghaus. 1993. Efficacy of a hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and diacetoxyscirpenol. Poult. Sci. 72:51-59.
Lightner, D.V. 1996. A Handbook of Shrimp Pathology and Diagnostic Procedures for Diseases of Cultured Penaeid Shrimp. World Aquaculture Society, Baton Rouge, LA, USA, p. 304.
McKean, C, L. Tang, M. Billam, M. Tang, C. W. Theodorakis, R. J. Kendall and J.-S. Wang. 2006. Comparative acute and combinative toxicity of aflatoxin B1 and T-2 toxin in animals and immortalized human cell lines. J. Appl. Toxicol. 26:139-147.
Oganesian, A, J.D. Hendricks, C.B. Pereira, G.A. Orner, G.S. Bailey and D.E. Williams. 1999. Potency of dietary indole-3-carbinol as a promoter of aflatoxin B1-initiated hepatocarcinogenesis: results from a 9000 animal tumor study. Carcinogenesis 20(3):453- 458.
Ostland V.E., H.W. Ferguson, R.D. Armstrong, A. Asselin and R. Hall. 1987. Granulomatous peritonitis in fish associated with Fusarium solani. Vet. Rec. 121(25-26):595-6.
Ramos, A.J., J. Fink-Gremmels and E. Hernandez. 1996. Prevention of toxic effects of mycotoxins by means of non-nutritive adsorbent compounds. J. Food Prot. 59:631-641.
Razzazi-Fazeli, E., J. Bohm, J. Grajewski, K. Szczepaniak, A. Kubber-Heiss and C. Iben. 2001. Residues of ochratoxin A in pet foods, canine and feline kidneys. J. Anim. Physiol. Anim. Nut. 85:212-216.
Rotter, R.G., A.A. Frohlich and R.R. Marquardt. 1989. Influence of dietary charcoal on ochratoxin A toxicity in leghorn chicks. Can. J. Vet. Res. 53:449-453.
Sahoo, P.K. and S.C. Mukherjee. 2002. Influence of high dietary α-tocopherol intakes on specific immune response, nonspecific resistance factors and disease resistance of healthy and aflatoxin B1-induced immunocompromised Indian major carp, Labeo rohita (Hamilton). Aquaculture Nutrition: 8(3):159-168.
Schaeffer, J.L. and P.B. Hamilton. 1991. Interactions of mycotoxins with feed ingredients. Do safe levels exist? In: Mycotoxins and Animal Foods (J.E. Smith and R.S. Henderson, eds). CRC Press, Inc., Boca Raton, FL, USA, pp. 827-843.
Shalaby, A.M.E. 2005. The opposing effect of ascorbic acid (vitamin C) on ochratoxin toxicity in Nile tilapia. In: Proc. 6th Int. Symp. on Tilapia Aquaculture (R. Bolivar, G. Mair and K. Fitzsimmons, eds). Publ. American Tilapia Association, Charlestown, VA, USA, pp. 209-221.
Shiau, S.Y., C.C. Kwok, C.J. Chen, H.T. Hong and H.B. Hsieh. 1989. Effects of dietary fibre on the intestinal absorption of dextrin, blood sugar level and growth of tilapia, Oreochromis nilotica × O. aureus. J. Fish Biol. 34(6):929-935.
Smith, T.K. 2005. Mycotoxins and adsorbents. Feed International 26(4).
Souheil, H., A. Vey, P. Thuet and J.-P. Trilles. 1999. Pathogenic and toxic effects of Fusarium oxysporum (Schecht.) on survival and osmoregulatory capacity of Penaeus japonicus (Bate). Aquaculture 178(3-4):209-224.
Spring, P. and D.F. Fegan. 2005. Mycotoxins - a rising threat to aquaculture. In: Nutritional Biotechnology in the Feed and Food Industries, Proceedings of Alltech’s 21st Annual Symposium (T.P Lyons and K.A. Jacques, eds). Nottingham University Press, UK, pp. 323-331.
Stangroom K.E. and T.K. Smith. 1984. Effect of whole and fractionated dietary alfalfa meal on zearalenone toxicosis and metabolism in rats and swine. Can. J. Physiol. Pharmacol. 62(9):1219-24.
Surai, P.F. and J.E. Dvorska. 2005. Effects of mycotoxins on antioxidant status and immunity. In: The Mycotoxin Blue Book (D. Diaz, ed). Nottingham University Press, UK, pp. 93- 137.
Van der Eijk, C. 2003. New technologies improve mycotoxin elimination. Feed Mix 11(1):8-11.
Whitaker, T.B., A.B. Slate and A.S. Johansson. 2005. Sampling feeds for mycotoxin analysis. In: The Mycotoxin Blue Book (D. Diaz, ed). Nottingham University Press, UK, pp. 1-23.
Wolf-Hall, C.E., M.A. Hanna and L.B. Bullerman. 1999. Stability of deoxynivalenol in heat-treated foods. J. Food Prot. 62:962-964.
Wyatt, R.D. 2005. Mycotoxin interactions. In: The Mycotoxin Blue Book (D. Diaz, ed). Nottingham University Press, UK, pp. 269-278.
Yiannikouris, A., L. Poughon, X. Cameleyre, C.-G. Dussap, J. Francois, G. Bertin and J.-P. Jouany. 2003. A novel technique to evaluate interactions between Saccharomyces cerevisiae cell wall and mycotoxins: application to zearalenone. Biotechnol. Lett. 25:783-789.
Yiannikouris, A., G. Andre, A. Buléon, G. Jeminet, L. Canet, J. Francois, G. Bertin and J.-P. Jouany. 2004a. Comprehensive conformational study of key interactions involved in zearalenone complexation with ß-D-glucans. Biomacromolecules 5:2176-2185.
Yiannikouris, A., J. Francois, L. Poughon, C.-G. Dussap, G. Bertin, G. Jeminet and J.- P. Jouany. 2004b. Adsorption of zearalenone by ß-D-glucans in the Saccharomyces cerevisiae cell wall. J. Food Prot. 67:1195-1200.
Yiannikouris, A., J. Francois, L. Poughon, C.-G. Dussap, G. Bertin, G. Jeminet and J.- P. Jouany. 2004c. Alkali-extraction of ß-D-glucans from Saccharomyces cerevisiae cell wall and study of their adsorptive properties toward zearalenone. J. Agric. Food Chem. 52:3666-3673.
Yiannikouris, A., G. Bertin and J.-P. Jouany. 2005. Reducing mycotoxin impact: the science behind Mycosorb®. In: Nutritional Biotechnology in the Feed and Food Industries, Proceedings of Alltech’s 21st Annual Symposium (T.P Lyons and K.A. Jacques, eds). Nottingham University Press, UK, pp. 265-276.
Yildirim, M., B. Manning, R.T. Lovell, J.M. Grizzle, and G.E. Rottinghaus. 2000. Toxicity of moniliformin and fumonisin B1 fed singly and in combination in diets for channel catfish. J. World Aqua. Soc. 31:599-608.
Zeijdner, E. and R. Havenaar. 2000. The fate of orally administrated compounds during passage through the gastrointestinal tract simulated in a dynamic in vitro model. European Pharmaceutical Contractor, Feb 2003:76-81.
Zekovic, D.B., S. Kwiatkowski, D. Jakovijevic and C. Moran. 2005. Natural and modified (1-3)-ß-D-glucans in health promotion and disease alleviation. Crit. Rev. Biotechnol. 25:205-230.
1 Alltech Inc., Bangkok, Thailand
2 Swiss College of Agriculture, Zollikofen, Switzerland






.jpg&w=3840&q=75)