Population Genetic Structure and Chemotype Diversity of Fusarium graminearum Populations from Wheat in Canada and North Eastern United States
Author details:

1. McMullen, M.; Jones, R.; Gallenberg, D. Scab of Wheat and Barley: A Re-emerging Disease of Devastating Impact. Plant Dis. 1997, 81, 1340–1348. [CrossRef]
2. Gilbert, J.; Tekauz, A. Review: Recent developments in research on fusarium head blight of wheat in Canada. Can. J. Plant Pathol. 2000, 22, 1–8. [CrossRef]
3. Summerell, B.A.; Laurence, M.H.; Liew, E.C.Y.; Leslie, J.F. Biogeography and Phylogeography of Fusarium: A Review. Fungal Divers. 2010, 44, 3–13. [CrossRef]
4. van der Lee, T.; Zhang, H.; van Diepeningen, A.; Waalwijk, C. Biogeography of Fusarium graminearum species complex and chemotypes: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 453–460. [CrossRef]
5. Sarver, B.A.; Ward, T.J.; Gale, L.R.; Broz, K.; Kistler, H.C.; Aoki, T.; Nicholson, P.; Carter, J.; O’Donnell, K. Novel Fusarium head blight pathogens from Nepal and Louisiana revealed by multilocus genealogical concordance. Fungal Genet. Biol. 2011, 48, 1096–1107. [CrossRef]
6. Amarasinghe, C.C.; Tittlemier, S.A.; Fernando, W.G.D. Nivalenol-Producing Fusarium cerealis Associated with Fusarium Head Blight in Winter Wheat in Manitoba, Canada. Plant Pathol. 2015, 64, 988–995. [CrossRef]
7. Kelly, A.C.; Clear, R.M.; O’Donnell, K.; McCormick, S.; Turkington, T.K.; Tekauz, A.; Gilbert, J.; Kistler, H.C.; Busman, M.; Ward, T.J. Diversity of Fusarium head blight populations and trichothecene toxin types reveals regional differences in pathogen composition and temporal dynamics. Fungal Genet. Biol. 2015, 82, 22–31. [CrossRef] [PubMed]
8. Henriquez, M.A.; Derksen, H.; Doherty, J.; Miranda, D.E.; Gruenke, O. Fusarium head blight of spring wheat in Manitoba in 2018. Can. Plant Dis. Surv. 2019, 99, 94–95.
9. Ward, T.J.; Clear, R.M.; Rooney, A.P.; O’Donnell, K.; Gaba, D.; Patrick, S.; Starkey, D.E.; Gilbert, J.; Geiser, D.M.; Nowicki, T.W. An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America. Fungal Genet. Biol. 2008, 45, 473–484. [CrossRef]
10. Codex DON MLs. Maximum Levels for DON in Cereals and Cereal Products: Update on Codex and Health Canada Activities. 2015. Available online: https://static1.squarespace.com/static/56be29e022482ec146a7c5b8/t/58406f5d6a496371af57670a/14 80617822134/CWFHB-S14-Elliott.pdf (accessed on 23 May 2020).
11. Clear, R.M.; Abramson, D. Occurrence of fusarium head blight and deoxynivalenol (vomitoxin) in two samples of Manitoba wheat in 1984. Can. Plant Dis. Surv. 1986, 66, 9–11.
12. Clear, R.M.; Patrick, S.K. Fusarium head blight pathogens isolated from fusarium-damaged kernels in western Canada 1993–1998. Can. J. Plant Pathol. 2000, 22, 51–60. [CrossRef]
13. Canada Grain Commission. Frequency and Severity of Fusarium Damaged Kernels in Wheat Samples from Manitoba, Saskatchewan and Alberta Crop Districts, 2003 to 2019. 2020. Available online: https://www.grainscanada.gc.ca/en/grainresearch/export-quality/cereals/wheat/western/annual-fusarium-damage/canada-western-red-spring/?wbdisable=true (accessed on 23 May 2020).
14. Henriquez, M.A.; Derksen, H.; Doherty, J.; Miranda, D.E.; Gruenke, O. Fusarium head blight of spring wheat in Manitoba in 2016. Can. Plant Dis. Surv. 2017, 97, 125–126.
15. Henriquez, M.A.; Derksen, H.; Doherty, J.; Miranda, D.E.; Gruenke, O. Fusarium head blight of spring wheat in Manitoba in 2017. Can. Plant Dis. Surv. 2018, 98, 121–122.
16. Rossi, V.; Ravanetti, A.; Pattori, E.; Giosuè, S. Influence of temperature and humidity on the infection of wheat spikes by some fungi causing fusarium head blight. J. Plant Pathol. 2001, 83, 189–198.
17. Fernando, W.D.; Oghenekaro, A.O.; Tucker, J.R.; Badea, A. Building on a foundation: Advances in epidemiology, resistance breeding, and forecasting research for reducing the impact of fusarium head blight in wheat and barley. Can. J. Plant Pathol. 2021, 1–32. [CrossRef]
18. Seed Manitoba. Manitoba Agriculture and Rural Development, Manitoba Seed Growers’ Association; Manitoba Co-Operator: Winnipeg, MB, Canada, 2020; Available online: https://www.seedmb.ca/digital-edition/seed-manitoba_2020-01-01/ (accessed on 19 May 2020).
19. Manitoba Agricultural Services Corporation (MASC). Variety Market Share Report. 2019. Available online: https://www.masc. mb.ca/masc.nsf/sar_varieties_2019.pdf (accessed on 19 May 2020).
20. Amarasinghe, C.; Sharanowski, B.; Fernando, W.G.D. Molecular Phylogenetic Relationships, Trichothecene Chemotype Diver-sity and Aggressiveness of Strains in a Global Collection of Fusarium graminearum Species. Toxins 2019, 11, 263. [CrossRef]
21. McDonald, B.A.; McDermott, J.M. Population Genetics of Plant Pathogenic Fungi. Bioscience 1993, 43, 311–319. [CrossRef]
22. Milgroom, M.; Fry, W. Contributions of Population Genetics to Plant Disease Epidemiology and Management. Adv. Bot. Res. 1997, 24, 1–30. [CrossRef]
23. Guo, X.W.; Fernando, W.G.D.; Seow-Brock, H.Y. Population Structure, Chemotype Diversity, and Potential Chemotype Shifting of Fusarium graminearum in Wheat Fields of Manitoba. Plant Dis. 2008, 92, 756–762. [CrossRef] [PubMed]
24. Burlakoti, R.R.; Tamburic-Ilincic, L.; Limay-Rios, V.; Burlakoti, P. Comparative Population Structure and Trichothecene Mycotoxin Profiling of Fusarium graminearum from Corn and Wheat in Ontario, Central Canada. Plant Pathol. 2017, 66, 14–27. [CrossRef]
25. Fernando, W.G.D.; Zhang, J.X.; Dusabenyagasani, M.; Guo, X.W.; Ahmed, H.; McCallum, B. Genetic Diversity of Gibberella zeae Isolates from Manitoba. Plant Dis. 2006, 90, 1337–1342. [CrossRef] [PubMed]
26. Tóth, B.; Mesterházy, Á.; Horváth, Z.; Bartók, T.; Varga, M.; Varga, J. Genetic variability of central european isolates of the Fusarium graminearum species complex. Eur. J. Plant Pathol. 2005, 113, 35–45. [CrossRef]
27. Liang, J.; Xayamongkhon, H.; Broz, K.; Dong, Y.; McCormick, S.; Abramova, S.; Ward, T.; Ma, Z.; Kistler, H. Temporal dynamics and population genetic structure of Fusarium graminearum in the upper Midwestern United States. Fungal Genet. Biol. 2014, 73, 83–92. [CrossRef]
28. Astolfi, P.; Reynoso, M.M.; Ramirez, M.L.; Chulze, S.N.; Alves, T.C.A.; Tessmann, D.J.; Del Ponte, E.M. Genetic population structure and trichothecene genotypes of Fusarium graminearum isolated from wheat in southern Brazil. Plant Pathol. 2012, 61, 289–295. [CrossRef]
29. Mahfooz, S.; Srivastava, A.; Srivastava, A.K.; Arora, D.K. A Comparative Analysis of Distribution and Conservation of Microsatellites in the Transcripts of Sequenced Fusarium Species and Development of Genic-SSR Markers for Poly-morphism Analysis. FEMS Microbiol. Lett. 2015, 362, fnv131. [CrossRef]
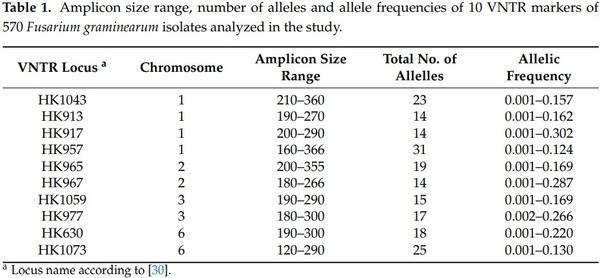
30. Suga, H.; Gale, L.R.; Kistler, H.C. Development of VNTR markers for two Fusarium graminearum clade species. Mol. Ecol. Notes 2004, 4, 468–470. [CrossRef]
31. Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [CrossRef]
32. Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [CrossRef]
33. Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [CrossRef] [PubMed]
34. Shah, D.A.; De Wolf, E.D.; Paul, P.A.; Madden, L.V. Functional Data Analysis of Weather Variables Linked to Fusarium Head Blight Epidemics in the United States. Phytopathology 2019, 109, 96–110. [CrossRef] [PubMed]
35. Vaughan, M.; Backhouse, D.; Del Ponte, E.M. Climate Change Impacts on the Ecology of Fusarium graminearum Species Complex and Susceptibility of Wheat to Fusarium Head Blight: A Review. World Mycotoxin J. 2016, 9, 685–700. [CrossRef]
36. Burlakoti, R.R.; Ali, S.; Secor, G.A.; Neate, S.M.; McMullen, M.P.; Adhikari, T.B. Genetic Relationships among Populations of Gibberella zeae from Barley, Wheat, Potato, and Sugar Beet in the Upper Midwest of the United States. Phytopathology 2008, 98, 969–976. [CrossRef] [PubMed]
37. Burlakoti, R.R.; Neate, S.M.; Adhikari, T.B.; Gyawali, S.; Salas, B.; Steffenson, B.J.; Schwarz, P.B. Trichothecene Profiling and Population Genetic Analysis of Gibberella zeae from Barley in North Dakota and Minnesota. Phytopathology 2011, 101, 687–695. [CrossRef]
38. Karugia, G.W.; Suga, H.; Gale, L.R.; Nakajima, T.; Tomimura, K.; Hyakumachi, M. Population Structure of the Fusarium graminearum Species Complex from a Single Japanese Wheat Field Sampled in Two Consecutive Years. Plant Dis. 2009, 93, 170–174. [CrossRef] [PubMed]
39. Puri, K.D.; Saucedo, E.S.; Zhong, S. Molecular Characterization of Fusarium Head Blight Pathogens Sampled from a Naturally Infected Disease Nursery Used for Wheat Breeding Programs in China. Plant Dis. 2012, 96, 1280–1285. [CrossRef]
40. Boutigny, A.–L.; Ward, T.J.; Ballois, N.; Iancu, G.; Ioos, R. Diversity of the Fusarium graminearum Species Complex on French Cereals. Eur. J. Plant Pathol. 2014, 138, 133–148. [CrossRef]
41. Aamot, H.U.; Ward, T.J.; Brodal, G.; Vrålstad, T.; Larsen, G.B.; Klemsdal, S.S.; Elameen, A.; Uhlig, S.; Hofgaard, I.S. Genetic and phenotypic diversity within the Fusarium graminearum species complex in Norway. Eur. J. Plant Pathol. 2015, 142, 501–519. [CrossRef]
42. Qiu, J.; Xu, J.; Shi, J. Molecular characterization of the Fusarium graminearum species complex in Eastern China. Eur. J. Plant Pathol. 2014, 139, 811–823. [CrossRef]
43. Qiu, J.-B.; Sun, J.-T.; Yu, M.-Z.; Xu, J.-H.; Shi, J.-R. Temporal Dynamics, Population Characterization and Mycotoxins Accumulation of Fusarium graminearum in Eastern China. Sci. Rep. 2016, 6, 36350. [CrossRef]
44. Maxwell, C.S.; Sepulveda, V.E.; Turissini, D.A.; Goldman, W.E.; Matute, D.R. Recent admixture between species of the fungal pathogen Histoplasma. Evol. Lett. 2018, 2, 210–220. [CrossRef]
45. Tamburic-Ilincic, L.; Wragg, A.; Schaafsma, A. Mycotoxin accumulation and Fusarium graminearum chemotype diversity in winter wheat grown in southwestern Ontario. Can. J. Plant Sci. 2015, 95, 931–938. [CrossRef]
46. Gale, L.R.; Ward, T.J.; Balmas, V.; Kistler, H.C. Population subdivision of Fusarium graminearum sensu stricto in the upper midwestern United States. Phytopathology 2007, 97, 1434–1439. [CrossRef]
47. Gale, L.R.; Harrison, S.A.; Ward, T.J.; O’Donnell, K.; Milus, E.A.; Gale, S.W.; Kistler, H.C. Nivalenol-type populations of Fusarium graminearum and F. asiaticum are prevalent on wheat in southern Louisiana. Phytopathology 2011, 101, 124–134. [CrossRef] [PubMed]
48. Bec, S.; Ward, T.; Farman, M.; O’Donnell, K.; Hershman, D.; Van Sanford, D.; Vaillancourt, L.J. Characterization of Fusarium Strains Recovered From Wheat With Symptoms of Head Blight in Kentucky. Plant Dis. 2015, 99, 1622–1632. [CrossRef]
49. Mishra, P.K.; Tewari, J.P.; Clear, R.M.; Turkington, T.K. Molecular genetic variation and geographical structuring in Fusarium graminearum. Ann. Appl. Biol. 2004, 145, 299–307. [CrossRef]
50. Keller, M.D.; Bergstrom, G.C.; Shields, E.J. The aerobiology of Fusarium graminearum. Aerobiologia 2013, 30, 123–136. [CrossRef]
51. Mishra, P.K.; Tewari, J.P.; Turkington, T.K.; Clear, R.M. Genetic evidences for a recent expansion of 15
52. Yörük, E.; Yli-Mattila, T. Class B-trichothecene profiles of fusarium species as causal agents of head blight. In Advancing Frontiers in Mycology and Mycotechnology: Basic and Applied Aspects of Fungi; Satyanarayana, T., Deshmukh, S.K., Deshpande, M.V., Eds.; Springer: Singapore, 2019; pp. 347–376.
53. Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; Pennsylvania State University Press: University Park, PA, USA, 1983.
54. Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006.
55. Nicholson, P.; Simpson, D.R.; Weston, G.; Rezanoor, H.N.; Lees, A.K.; Parry, D.W.; Joyce, D. Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiol. Mol. Plant Pathol. 1998, 53, 17–37. [CrossRef]
56. Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [PubMed]
57. Earl, D.A.; Vonholdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2011, 4, 359–361. [CrossRef]







