Penicillic Acid
Published: May 10, 2018
By: Sergio Paulo Severo de Souza Diniz / Associate Professor Retired - Department of Biochemistry of the State University of Maringá (UEM), Maringá, PR - Brazil. Website: www.spdiniz.com.br.
Introduction
The penicillic acid was isolated from maize infected with Penicillium puberulumin in 1913. Currently, it is known that it is also produced by other members of the genus Penicillium and Aspergillus. As for toxicity, is less toxic than patulin, although keep structural similarities, both are -lactones, which explains their strong carcinogenic activity. It is considered a potent antimicrobial agent. It is a toxin tremorgenic being the main symptoms of poisoning, tremors and convulsions.

Figure 1- Giant colony of Penicillium in culture medium. (Source: Carlos Antonio Locatelli)
Corn silages were inoculated with strains of Penicillium roqueforti, which are capable of producing the following mycotoxins: penicilic acid (PA), mycophenolic acid (MPA), patulin (PAT) or PR toxin (PRT). The silage was incubated for 160 days in aerobic conditions, without agitation at 15ºC in the dark. Maximal levels were found in mg kg-1as follows: 3.56 mycophenolic acid; patulin 15.10; acid penicilic 3.06, and 2.17 toxin PR. This production was preceded by increasing the pH from 4 to 9 (Muller & Amend, 1997).
Formerly in 1996, Lopez Diaz et al., studied the presence of mycotoxins in varieties of cheeses in Spain. They recorded the occurrence of aflatoxin B-1 and M-1, esterigmatocistina, patulin, penicilic acid and mycophenolic acid. In parallel, we isolated 24 strains of Aspergillus and Penicillium. A total of 84 samples medicinal plants were investigated, contaminated by molds and mycotoxins (Aziz et al.1998). Were detected 10 genera of fungi microorganisms of different groups taxonomics and most frequent were Aspergillus flavus, A. parasiticus, A. niger, Penicillium viridicatum and Fusarium oxysporum. Aflatoxin B-1 were found in 17 samples (10-160 µg Kg-1); ochratoxin A in 3 samples, P. viridicatum, P. chrysogenume and P. commune demonstrated to be penicillic acid producers. The P. cyclopium may produce more than 20 toxic metabolites, among which were identified ochratoxin A (5), penicillin acid (Birkinshaw, 1936), cyclopiazonic acid (Holzapel, 1968), and penitrems A and B (Hou et al., 1971). P. canescens was less studied but produced: citrinin (8) and canescin (3). These mycotoxins, penicillin acid was reported as inhibitor of growth of young plant roots of rice (Sassa et al., 1971) and of oat seedlings by lessening their respiration (Bastin & Roey, 1954). It was also reported as urease inhibitor (Reiss, 1979) and RNase activity (Tashiro et al., 1979). Was demonstrated the phytotoxic effect of the culture filtrates of P. cyclopium and P. canescens on the germination of corn seeds and isolated and identified penicillic acid as a responsible agent (Keromnes & Thouvenot, 1985).
First isolation
The first material was isolated from corn infected with Penicillium puberulum by Alsberg and Black in 1913 by the Department of Agriculture of the United States of America.
Fungi producers
Aspergillus melleus, Aspergillus ochraceus, Penicillium puberulum Penicillium cyclopium, Penicillium thomii, Penicillium suaviolens, Penicillium boarnense, Penicillium olivino-viriall, Penicillium mortersii, Penicillium fenelliae, pulitans Penicillium, among others.
Susceptible food
Wheat, flour, bread, corn flour, corn, popcorn, beans, soybeans, sorghum, barley, meat pies, cocoa powder, cheese, salami, sausage, ham, cured, moldy meat, chilled and frozen sweets, moldy foods supermarket and refrigerated foods stored at home or not.
Sensitive animal
Animals in general, especially those that feed ratio of corn and the like, and men.
Symptoms
The penicillin acid is carcinogenic, cytotoxic, hepatotoxic, dilates coronary and pulmonary arteries, tremors, convulsions, Slobber syndrome, excessive salivation. However, it is considered a potent antimicrobial agent.
Chemical characteristics
- Molecular formula: C8H1004;
- Molecular weight: 170.16 g mol-1;
- Name: 3-Methoxy-5-methyl-4-oxo-2 ,5-hexadienoic;
- Physical properties: Crystal rhombic, monoclinic or triclinic in water (monohydrate);
- Melting point: 83-84° C;
- Emits fluorescence: transparent;
- Refractive index: uv max 200 nm;
- Solubility: Reaction acid becomes blue red, moderately soluble in cold water, soluble in hot water, alcohol, ether, benzene, chloroform.
- LD50: 100 mg kg-1for mice
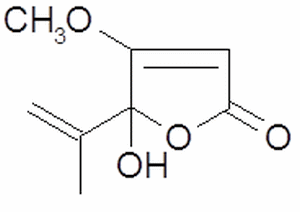
Figure 2 - Molecular estructure of Penicilic acid
References
Bastin, R., and G. Van Roey. 1954. Action of some antibiotics and inhibiting substances on cellular stretching. P. Pharm. Belg. 9:112-127.
Birkinshaw, J. H., A. E. Oxford, and H. Raistrick. 1936. Penicillic acid, a metabolic product of Penicillium puberulum Banier and Penicillium cyclopium Westling. Biochem. J. 30: 394-411.
Holzapfel, C. W. 1968. The isolation and structure of cyclopiazonic acid a toxic metabolite of Penicillium cyclopium Westling.Tetrahedron 24:2101-2119.
Hou, C. T., A. Ciegler, and C. W. Hesseltine. 1971. Tremorgenic toxins from penicillia. III. Tremortin production by Penicillium species on various agricultural commodities. Appl. Microbiol. 21:1101-1103.
Keromnes, J. & Thouvenot, D. 1985.Role of Penicillic Acid in the Phytotoxicity of Penicillium cyclopium and Penicillium canescens to the Germination of Corn Seeds.Appl. Environ. Microbiol., v. 49, p. 660-663.
LopezDiaz, T.M.; Roman-Blanco, C.; Garcia-Arias, M.T.; Garcia-Fernandez, M.C.; Garcia-Lopez, M.L. 1996. International J. Food Microbiology v.30, n.3, p.391-395.
Muller, H.M. E Amend, R. 1997. Formation and disappearance of mycophenolic acid, patulin, penicillic acid and PR toxin in maize silage inoculated with Penicillium roqueforti. Arch.Animal Nutrition v.50, n.3, p. 213-225.
Reiss, J. 1979. Inhibitory action of the mycotoxins patulin and penicillic acid on urease.Food Cosmet.Tocicol. 17:145-146. 24.
Sassa, T., S. Hayakari, M. Ikeda, and Y. Miura. 1971. Plant growth inhibitors produced by fungi. I. Isolation and identification of penicillic acid and dihydropenicillic acid. Agric. Biol. Chem. 35:2130-2131.
Tashiro, F., K. Hirai, and Y. Ueno. 1979. Inhibitory effects of carcinogenic mycotoxins on deoxyribonucleic acid-dependent ribonucleic acid polymerase and ribonuclease H. Appl. Environ. Microbiol. 38:191-196.
spssDiniz – April 2, 2018
Related topics:
Authors:

Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.




