mycotoxin contamination of silages
Mould growth and mycotoxin contamination of silages: sources, types and solutions
Introduction
Silages made from a variety of forage crops constitute important components of ruminant diets in many areas of the world. New estimates for Germany, for example, indicate that silage production is the biggest on-farm preservation operation (Weissbach, personal communication). In 2000, a total of 76.2 million tonnes of silage were produced compared to only 2.3 tonnes of hay (based on fresh matter). Maize silage represents the major proportion (about 58%), followed by grass and legume silage (about 32%) and whole-crop cereal silage (about 2%).
In order to ensure good animal health and performance, it is essential to produce silages with high feeding value and good hygienic quality. Apart from contamination of silages with undesired or even
pathogenic microorganisms, e.g., Clostridium tyrobutyricum, Clostridium botulinum, Listeria monocytogenes and Escherichia coli O157 (Woolford, 1990; Fenlon and Wilson, 2000; Thylin, 2000), the occurrence of filamentous fungi (moulds) and their secondary toxic metabolites (mycotoxins) have attracted considerable attention as potential causes for poor performance and health disorders in domestic livestock (Nibbelink, 1986; Dieckman and Green, 1992; Diaz and Boermans, 1994; Marquardt, 1996; Bauer, 2002). In comparison to cereal grains and proteinaceous feed materials, however, comprehensive knowledge of moulds and mycotoxins in silages and their effects on animal production is still lacking (Scudamore and Livesey, 1998).
The term ‘moulds’ refers to a diverse group of microorganisms that are ubiquitous in nature and exist as saprophytes or plant pathogens. The genera Fusarium, Alternaria, Cladosporium, Pencillium and Aspergillus are often considered the most prominent feed-borne filamentous fungi (Lacey and Magan, 1991; Auerbach and Geissler, 1992; Miller, 1995; Scott, 2001).
Mycotoxins are fungal metabolites, representing a great variety of chemical families and a range of molecular weights. There are several hundreds known (Cole and Cox, 1981; Steyn, 1998), but few have been extensively studied. Mycotoxins that are potential contaminants of forage crops and silages prepared from them, as well as their main producers are summarized in Table 1 (Scudamore and Livesey, 1998).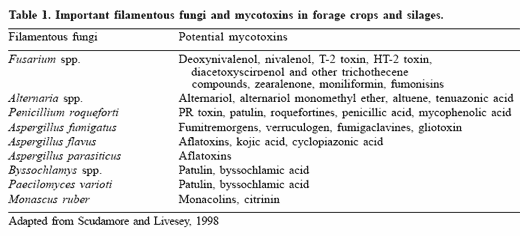
Most of the research has been conducted on aflatoxins, ochratoxins, trichothecenes, fumonisins and zearalenone. Mycotoxins exhibit many different biological effects in the animal. They can be carcinogenic, teratogenic, genotoxic, hepatotoxic, nephrotoxic, haematotoxic, immunosuppressive, estrogenic, tremorgenic or mutagenic (Trenholm et al., 1997; Dirheimer, 1998; Oswald and Comera, 1998; Parent-Massin and Parchment, 1998; Riley et al., 1998; Shier, 1998; Steyn, 1998). Figure 1 indicates where several mycotoxins interfere with carbohydrate metabolism (Kiessling, 1986).
Although it is well established that a significant proportion of cereal crops worldwide is affected by mycotoxins annually and results in economic losses (CAST, 1989; Moss, 1991), the contamination of forage crops and silages with toxic fungal metabolites has been largely ignored (Smith et al., 1994). Therefore, this article reviews available information on factors affecting growth and mycotoxin formation by filamentous fungi in silages as well as their presence and significance in commercial silages. Measures to reduce the risks associated with moulds and mycotoxins in silages are briefly described.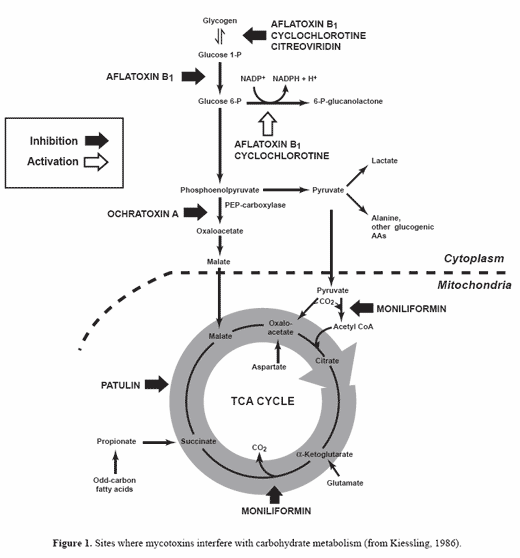
Moulds in silages
FUNGAL DYNAMICS DURING FERMENTATION AND SUBSEQUENT FEEDOUT OF SILAGES
Mould growth is determined by several environmental factors that markedly affect the composition of the mycoflora in feeds. Of particular importance are temperature, composition of the gas atmosphere, substrate properties including moisture content and water activity (aw), pH and chemical composition, as well as biotic factors (insects, vertebrates and other microorganisms) (Ramakrishna et al., 1993; Ominski et al., 1994). The general requirements of filamentous moulds for growth have been reviewed in detail by Lacey and Magan (1991). Complex interactions between these determinants make it very likely that the mycoflora on growing crops will differ significantly from that of stored products (Lacey, 1989; Miller, 1995; Petersson and Schnürer, 1995).
As reported for lactic acid bacteria and yeasts (Jonsson and Pahlow, 1984; Chunjian et al., 1992), the population of filamentous fungi also undergoes significant changes between pre-ensiling on growing crops through silage fermentation to feedout of the silages (Pelhate, 1977). In the field, forage crops harbour mycoflora mainly represented by species of the genera Fusarium, Cladosporium and Alternaria, but also some storage moulds, such as Penicillium and Aspergillus, may be found (Niles, 1980; Müller, 1991; Auerbach and Geissler, 1992; Miller, 1995). The availability of oxygen plays a crucial role in the development of a characteristic silage mycoflora. When oxygen becomes depleted during the initial stages of fermentation, Fusaria can no longer survive (Damaglou et al., 1984; Lepom et al., 1988). Alternaria and Cladosporium soon disappear as well. Delayed sealing, however, will ultimately result in an increase of the mould count in silages at the early stages of ensilage (Mills and Kung, 2002).
According to Pelhate (1977), who classified filamentous fungi in silages on the basis of their tolerance to oxygen deprivation, Fusarium species are strictly aerobic. Tolerant moulds include A. fumigatus, several Mucorales and Penicillia as well as Monascus ruber. Some Mucor species, B. nivea, P. varioti and P. roqueforti are considered micro-aerophilic or indifferent to oxygen presence. In a series of trials with grass and whole-crop maize, Auerbach (1996) showed a continuous decline in mould count if the ensiled material was stored under anaerobic conditions from the beginning of ensilage. Air penetration at the initial stages of fermentation supported the growth of several mould species before their numbers decreased. Under any of the oxygen status conditions, P. roqueforti was the only filamentous fungus found after 60 days of storage (Figure 2).
In contrast, permanent oxygen infiltration did not reduce the level of moulds over the entire experimental period, and species other than P. roqueforti were frequently detected (Figure 3). Monascus was also shown to survive for longer periods in silages in the absence of oxygen (Auerbach, unpublished).
Another factor that may contribute to the succession of silage mycoflora during fermentation is pH changes caused by the natural production of organic acids, such as lactic, acetic, propionic and butyric acids. Although pH per se does not detrimentally affect filamentous fungi, as they can grow or lie dormant over a wide range between pH 3 and 8, variation in this parameter may have an influence on their susceptibility to other environmental factors (Lacey, 1989). The resistance of fungal conidia towards organic acid has been shown to differ among genera and species (Fencl and Leopold, 1957; Müller et al., 1981). Lactic acid has no detrimental effect (Woolford, 1975; El-Gazzar et al., 1987), whereas propionic acid and butyric acids are potent mould inhibitors. Conidia of P. roqueforti proved to be less impaired by propionic acid than those of other Penicillia or Aspergilli (Poisson and Cahagnier, 1973).
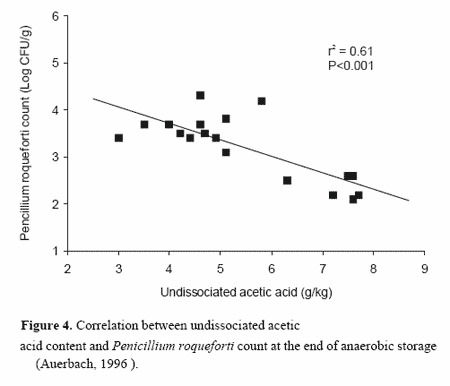
However, it is only the pH-dependent undissociated forms of organic acids that affect fungi as they are able to passively penetrate cell structures (hyphae and conidia). The degree of dissociation declines with decreasing pH (Lück, 1985). Investigations by Auerbach (1996) revealed a significant negative correlation between the content of undissociated acetic acid and the P. roqueforti count at the end of anaerobic storage (Figure 4). A low spore load prior to opening the silo is beneficial to improving aerobic stability of silages (Rust and Yokoyama, 1992).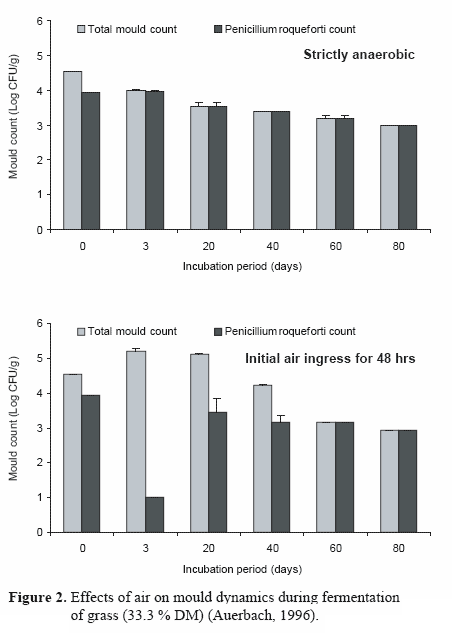
During feedout, oxygen will ultimately gain access to the silage and enable significant growth of filamentous fungi unless other factors, such as temperature, content of antimycotic organic acids, substrate composition and competitiveness, limit their development. All micro-aerophilic species have the advantage that they can begin to proliferate earlier, at lower oxygen and higher carbon dioxide concentrations. P. roqueforti requires a minimal oxygen concentration of 4.2% for growth if the carbon dioxide content of the environment does not exceed 80% (Moreau, 1981). B. nivea and B. fulva are known to grow in carbon dioxide-laden atmospheres with as little as 0.27 % oxygen (Lacey, 1989). By utilizing oxygen and carbon sources, certain filamentous fungi may potentially suppress the growth of other moulds even if they had survived the fermentation process (Parsons, 1991).
Substrate composition exerts a pronounced effect on fungal ecology. P. roqueforti and A. fumigatus grow well in aerobically stored silages (Tüller et al., 1995; Auerbach, 1996; Müller and Amend, 1997). It is accepted that organic acids markedly affect the composition of the mycoflora (Clevström et al., 1989). Compared to other moulds, P. roqueforti can proliferate in the presence of higher concentrations of organic acids (Engel and Teuber, 1978; Auerbach, 1996). Since these compounds represent a significant proportion of the total utilizable carbon sources in fermented crops, the development of moulds, which not only grow in their presence but can also metabolize them, might be favoured. The ability to use organic acids as a substrate has been demonstrated for a variety of moulds, including A. glaucus, P. varioti, P. roqueforti, P. cyclopium, A. flavus, P. expansum, A. candidus (Richards and Lloyd, 1966; All-Hilli and Smith, 1979; Lord et al., 1981; Müller and Hörber, 1982, Vivier et al., 1992; Auerbach, 1996). Treatment of crops with silage additives prior to ensiling was shown not only to influence fungal dynamics during fermentation, but also to have an effect on microbial ecology in silages upon subsequent exposure to air (Jonsson et al., 1990; Inglis et al., 1999).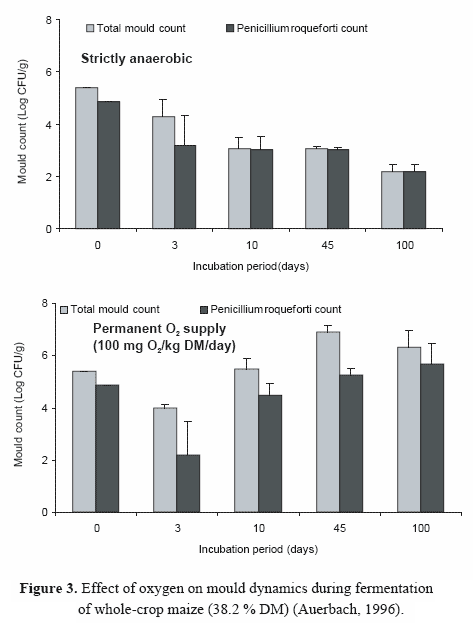
TYPES OF MOULDS IN SILAGES
In a survey in France and Italy in three successive years, Pelhate (1975; 1977) analysed the mycoflora of 1230 maize silage samples from commercial farms and isolated a non-exhaustive count of 70 fungal species. However, P. roqueforti was the dominating mould, present in 76% of the samples. The incidence of the genera Monascus, Aspergillus, Byssochlamys and Paecilomyces was 31%, 21%, 41% and 27%, respectively. Mucoraceae were also present in abundance. Comparable results were found by Escoula et al. (1972).
Vesely et al. (1981) observed the prevalence of P. roqueforti in silages in the Czech Republic, particularly during the winter feeding period. In addition, under Japanese conditions this filamentous fungus has been noted to occur at a high frequency (Nakane, 1946; Tubaki, 1956; Ohomo and Kitamoto, 1994).
The study by Gedek et al. (1981) included 260 farm silages in Germany and showed a high degree of contamination with P. roqueforti and A. fumigatus. These species were detected in 71.3 and 13.3% of the samples, respectively. Mucor, Absidia, Rhizopus, other Penicillia and Aspergilli as well as Monascus, Byssochlamys and P. varioti were less frequently isolated.
Amend (1990) studied the composition of the maize silage mycoflora in farm silages (whole-crop maize and corn-cob mix) from southern Germany and found P. roqueforti to be the most abundant species. Also in Germany, Frevel et al. (1985) detected P. roqueforti in 38.8% of the tested silages. Mucor and Absidia species were isolated from 36.4% of the samples. The genera Monascus, Scopulariopsis, Byssochlamys and Paecilomyces as well as Aspergillus were found in 12.4%, 11.6%, 10.1% and 9.3% of the silages, respectively. Fusaria were present in less than 1% of the samples. A mycological survey of 98 grass silage and 135 maize silage samples collected during 1997 and 1998 in southern Germany clearly indicated the predominance of three major species. P. roqueforti occurred in 30% of the silages, and M. ruber and A. fumigatus were present in 19% and 9% of the samples (Schneweis et al., 2000). Another investigation into the composition of the mycoflora of silages in southern Germany showed the prevalence of P. roqueforti, which was isolated from 27% of the samples. Species of minor incidence were Rhizopus nigricans, A. fumigatus and M. ruber (Armbruster, 1994).
Grass and maize silages from northern Germany were mainly infested with P. roqueforti (Auerbach et al., 1998, Table 2). Other moulds identified belonged to the genera Aspergillus, Mucor, Monascus and Geotrichum.
Of 455 Austrian grass and maize silages analyzed for filamentous fungi, 53.6% contained P. roqueforti, B. nivea, A. glaucus and M. ruber, respectively. Absidia, Mucor and Rhizopus were detected in 6.4% of the samples only (Adler, 1993). A Dutch survey on silages from sugar beet press pulp and maize revealed no marked differences in the mycological state between the silage types. P. roqueforti was isolated from 40% of the samples. Species of the genera Mucor, Byssochlamys and Geotrichum, A. fumigatus, M. ruber were found at a frequency of 23%, 4%, 8%, 8% and 4%, respectively (Nout et al., 1993). Recent studies carried out in Scandinavia confirmed the dominance of P. roqueforti in silages in this region as well (Skaar, 1996; Sundberg and Häggblom, 1999). A. fumigatus is commonly reported in silages in the US (Cole, 1976; Cole et al., 1977), and may be detected especially in the upper layers of the silo (Dutkiewicz et al., 1989). A mycological survey of Indian animal feedstuffs, including silages, showed that Aspergillus species (e.g. A. flavus, A. parasiticus, A. fumigatus) accounted for 19.3% of the total fungal isolates. However, the remaining proportion of the mycoflora was not described (Mor and Singh, 2000).
The variable results on the mycoflora in silages with respect to presence, abundance and dominance of certain fungal species may be attributed to a number of factors. The methods employed in the laboratory analysis of filamentous fungi may have an effect. Pelhate (1977) pointed out that incubation under anaerobic or aerobic conditions influences the range of species cultured. Temperature and composition of the growth medium play an important role as well (Jarvis et al., 1983; Skaar and Stenwig, 1996).
Evidence suggests that fungal populations may differ with silage type. Frevel et al. (1985) noted a more diverse mycoflora in grass silages than in maize silages. Furthermore, Armbruster (1994) showed that the predominantly-occuring Penicillia were significantly less frequent if Monascus species were present. The author associated these findings with the production of an inhibitory substance by Monascus which was, however, not determined. The temperature at sampling, the location in the silo from which the silage was removed (surface or deeper layers, cutting face), the visual appearance of the sample (visibly moulded or unmoulded) as well as the stage of aerobic deterioration at sampling have been addressed as potential causes for the variability of the data in the literature (Auerbach, 1996).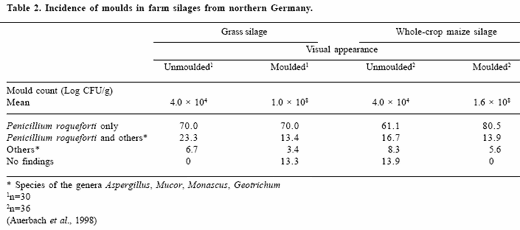
Mycotoxins in silages
PRESENCE OF MYCOTOXINS DERIVED FROM THE FIELD
Many mycotoxins found in silage are produced in the field prior to harvest and ensiling. Almost any part of the growing plant can be contaminated. Depending on the ecology of the mould, leaves, husks, kernels or the whole plant can contain mycotoxins. As most silage types are made from the whole plant (e.g., whole-crop maize and wholecrop cereals) the infection of any part of the plant may result in mycotoxin contamination of the respective silage.
The presence of toxic fungal metabolites in grains derived from the field, such as aflatoxins, fumonisins, trichothecenes, zearalenone and Alternaria toxins (alternariol, alternariol monomethyl ether, altuene
and tenuazonic acid) has been reported in numerous studies, and the co-occurrence of various mycotoxins is likely to be found (Müller et al., 1997; Scudamore et al., 1997; Scudamore et al., 1998; Scott, 2001; Whitlow and Hagler, 2002). Oldenburg (1999) summarized data on the occurrence of mycotoxins in forage crops. Several trichothecenes (e.g. deoxynivalenol (DON)) and zearalenone were found in grasses at concentrations up to approximately 2 mg/kg. Forage maize also contained DON and zearalenone at varying levels, between 0.005 and 13.75 mg/kg DM. The incidence of these mycotoxins and their concentrations were markedly affected by the plant fraction analyzed. It was interesting to note that maize cobs contained zearalenone less frequently and at lower concentrations than was determined for the stover. Oldenburg (1996) found a highly significant correlation between the DM content of the stover at harvest and the zearalenone content (Figure 5). Forage maize has been reported to contain ochratoxin A (OTA) prior to harvest (Oldenburg, 1991). As toxigenic Alternaria represent a frequently-occurring mould genus on growing forage maize, especially on leaves and husks (Müller, 1991; Müller, 1992), the presence of their toxins is likely.
Fungal infestation and mycotoxin formation in crops before harvest is a very probable explanation for the contamination of silages with field-derived toxins because, under appropriate ensiling conditions, the toxin-producing species are normally replaced by the characteristic silage mycoflora during the early stages of fermentation.
Gotlieb (1997) summarized the results of a number of surveys in the US, indicating a high incidence of DON in silages at levels of up to 3 mg/kg. In Germany, zearalenone, DON and OTA were frequently isolated from whole-crop maize silages, maize ear silages and corn-cob-mix (Kämpfe, 1999). The concentrations in silage varied between 33 and 51 ppb for zearalenone, 673-4297 ppb for DON and 17-37 ppb for OTA. A review by Oldenburg (1999) revealed that grass and maize silages may be contaminated with a range of trichothecenes and zearalenone. Mycotoxin contents were stated to be in the lower ppm range.
Whitlow and Hagler (2002) analyzed maize silages from farms in North Carolina and found a variety of toxic fungal metabolites that were likely to have originated from the field. The incidence differed with the mycotoxin tested. Mean concentrations for aflatoxins, DON, zearalenone and T-2 toxin were 28 ppb, 525 ppb, 1,991 ppb and 569 ppb, respectively. Fumonisins were also frequently detected, but the respective levels were not given. Maize silage from Mexico was contaminated with aflatoxin at concentrations between 500 and 5000 ppb (Rosiles, 1978).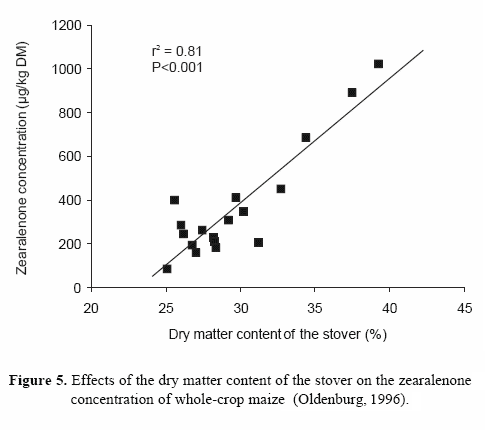
FATE OF FIELD-DERIVED MYCOTOXINS DURING FERMENTATION
The fate of field-derived mycotoxins in silages is still not fully understood. It is, however, assumed that these metabolites possess high stability under acidic conditions (Müller, 1983). A decline in zearalenone and DON concentrations was not observed during the course of fermentation of maize (Lepom et al., 1988, Lepom et al., 1990). In a series of laboratory ensiling trials with grass and maize, Oldenburg (personal communication) found no evidence of a substantial effect of fermentation on DON content. The mean initial concentration of this mycotoxin was 570 ppb, and after a prolonged period of fermentation an average concentration of 620 ppb DON DM was calculated. However, Hacking (1979) noticed that pre-formed zearalenone only remained stable for a very short period of time in corn and cob mixes. Aflatoxin formed in maize prior to ensiling has been shown to break down slowly (Kalac and Woolford, 1982). Lindenfelser and Ciegler (1970) studied the fate of aflatoxin during ensilage and found little or no reduction in the mycotoxin content after 26 days of storage. Inactivation of aflatoxin could be achieved by addition of lactic and hydrochloric acids. The effect was strongly related to the acid used, as well as to acid concentration and period of treatment, with hydrochloric being more effective.
Biodegradation of mycotoxins by microorganisms has been frequently reported (Smith and Harran, 1993). Degradation of mycotoxins during ensilage however is largely a matter of speculation. Böhm et al. (1999) provided evidence that the trichothecenes nivalenol and DON as well as OTA, zearalenone and fumonisins can be degraded in vitro by yeasts of the genera Saccharomyces, Kluyveromyces and Rhodotorula. The degradation capacity differed with the strain and the mycotoxin studied. Furthermore, certain lactic acid bacteria strains of the species Lactobacillus salivarius, Lactobacillus plantarum and Lactobacillus lactis decreased fumonisin concentration in the medium in vitro when co-cultured with F. moniliforme, a potent producer of this mycotoxin. It was not proven if the lactic acid bacteria suppressed the formation of fumonisin or degraded the toxin produced in the medium. Whether these findings can be applied to complex microbial ecosystems in silages remains to be clarified.
FORMATION OF MYCOTOXINS IN SILAGES
The detection of filamentous fungi in silages is not definite proof of the presence of mycotoxins. As previously described for fungal growth, mycotoxin formation is also affected by a complex of environmental factors (Lacey and Magan, 1991; Ramos et al., 1998). As not all strains of a given mould species are capable of elaborating mycotoxins, the development of toxigenic strains in silages is the prerequisite for the production of toxic metabolites in these feeds. Although mycotoxin formation in vitro on synthetic media appears to be a common characteristic among silage moulds, caution should be taken as to whether this ability is to be observed in situ.
Gedek et al. (1981) showed the production of PR toxin by all 37 P. roqueforti isolates tested. Adler (1993) found a high incidence of formation of mycophenolic acid and PR toxin in P. roqueforti strains from silages.
Results obtained for Paecilomyces strains from conserved forages provided evidence that toxigenicity is frequently observed in this species. Of a total of 26 isolates, 21 were able to produce patulin (Hacking and Rosser, 1981). Escoula and Henry (1975) established that 6 of 10 strains of B. nivea, all four strains of B. fulva and 3 of 6 strains of P. varioti, elaborated byssochlamic acid in pure culture at varying concentrations.
P. verrucosum strains found in silages exhibited toxigenic potential in vitro. 32.1% of the 28 isolates elucidated penicillic acid (Gedek et al., 1981). Of 13 toxigenic A. fumigatus species derived from fermented forages, 46.2% produced fumitremorgen B and C, verruculogen and TR-2 toxin simultaneously. Furthermore, fumitremorgen C and TR-2 toxin were elicited by 53.8% of these isolates (Gedek et al., 1981). Cole et al. (1977) identified several mycotoxins belonging to the fumitremorgen group in a culture of an A. fumigatus strain from silage. Among 10 A. flavus selected from silages, nine produced kojic acid, and one simultaneously produced cyclopiazonic acid and kojic acid (Gedek et al., 1981). In another study, the incidence of aflatoxin-producing A. flavus and A. parasiticus from silages was determined to be 9.1% and 18.2%, respectively (Mor and Singh, 2000).
When air penetrates into the silage after opening the silo, mould growth and mycotoxin production may be initiated. It was shown for P. roqueforti and A. fumigatus that significant concentrations of roquefortine C as well as verruculogen and fumitremorgen B can be formed in grass and maize silages (Tüller et al., 1995; Auerbach, 1996; Figures 6 and 7). Evidence is provided that production and accumulation rate of toxic metabolites differ with substrate (Olivigni and Bullermann, 1977) and mycotoxin produced. It is very likely that the type of available carbon sources and their utilizability may better support the growth of P. roqueforti and formation of roquefortine C in maize silage when compared with wilted grass silage. These silages often have excess sugar, and moulds grow twice as fast on sugars as on fermentation products (Muck and Bolsen, 1991; Pitt et al., 1991).
Müller and Amend (1997) studied the accumulation of mycophenolic acid, patulin, penicillic acid and PR toxin in maize silage inoculated with P. roqueforti over 160 days of aerobic storage. All mycotoxins were formed at varying concentrations, reaching maximum levels of 3.56 ppm mycophenolic acid, 15.1 ppm patulin, 3.06 ppm penicillic acid and 2.17 ppm PR toxin. It was noted that mycotoxin concentrations decreased below the limit of detection after an extended experimental period. Chemical reactions of those compounds with ammonia, amines, free amino acids and enzymes containing sulfhydryl (SH) groups were discussed as causes for the decline in mycotoxin levels, rendering them undetectable by the methods used (Wei et al., 1973, Lieu and Bullerman, 1978; Amend, 1990). Interactions between patulin and the yeast flora have also been addressed as potential causes for the decline in mycotoxin concentration in the course of fermentation. There was an inverse relationship between the number of yeasts and the patulin level (Dutton et al., 1984).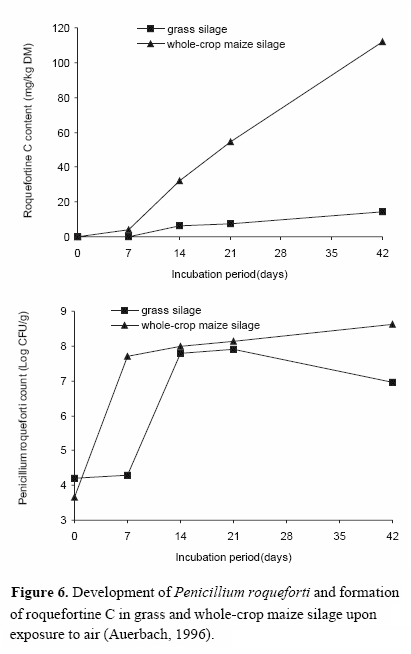
Commercial silages may frequently be contaminated with mycotoxins. Escoula (1974) collected 25 samples form the cutting face of maize silages and found patulin in 15 samples at levels of 2-40 ppm. A high concentration of PR toxin (8 ppm) was detected in silage from the US (Scott, 1981). These results were not confirmed by Müller and Amend (1997), who found no patulin or PR toxin in commercial maize silage samples.
Of 39 visibly moulded maize silage samples from southern Germany, five samples contained penicillic acid at 0.23-2.1 ppm, and five were contaminated with 0.1-4.2 ppm mycophenolic acid (Müller and Amend, 1997). Adler (1993) tested Austrian farm silage samples for mycophenolic acid and found levels up to 80 ppm. More recent studies by Schneweis et al. (2000) confirmed the high incidence of mycophenolic acid in commercial silages. Of a total of 233 grass and maize silage samples, 31.8% contained this mycotoxin. The mean concentration was 1.4 ppm, ranging between 0.02 and 35 ppm.
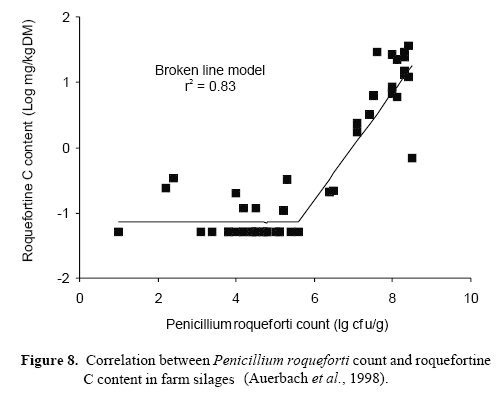
The incidence of roquefortine C, a secondary metabolite of P. roqueforti, in commercial silages has received considerable attention. Adler (1993) detected up to 5 ppm roquefortine C in these feedstuffs. Differences in the maximum roquefortine C contents between silages made from grass, maize cobs and whole-crop maize were noted by Armbruster (1994). They ranged between 0.099-0.58 ppm for grass silages, 0.086-2.1 ppm for corn-cob mix and 0.047-28.15 ppm for whole-crop maize silages. These findings were supported by Auerbach et al. (1998), additionally indicating an effect of the stage of deterioration on the incidence and concentration of roquefortine C (Table 3; Figure 8). Schmerbauch et al. (1999) found roquefortine C only in a few bale silages from grass. The highest level of roquefortine C was 0.35 mg/kg DM.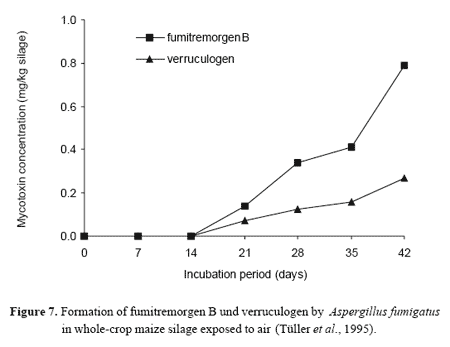
More recently, Schneweis et al. (2001) reported the occurrence of monacolins and citrinin, known to be produced by M. ruber, in 233 silages from southern Germany. It is very likely that this mycotoxin is formed in silages as the species can survive the ensiling process and may then show significant growth during feedout. Monacolins can be present in two chemical forms, as acid (monacolin A) or lactone (monacolin L). Considering all silages, 22% were contaminated with monacolins. Monacolin A concentrations ranged between 28 and 65,400 ppb, averaging 4,379 ppb. The level of monacolin L varied between 25 to 15,600 ppb, and a mean concentration of 1,775 ppb was calculated. The analysis for citrinin showed that 6% of the samples contained this mycotoxin at a level of up to 64.2 ppb.
Prevention of mould and mycotoxin contamination of silages
Agronomic measures play a key role in the control of mould growth and production of toxic metabolites on growing forage crops and therefore can significantly affect the amount of field-produced toxins in silage (Scott, 1988; Trenholm et al., 1989; Ellis et al., 1991; Miller, 1995 and Oldenburg et al. (2000). Due to genetic differences in susceptibility to fungal colonization, careful selection of crop varieties and use of sound, fungus-free seed form the basis of producing plant material of low mycotoxin contamination. The incidence of various moulds and their mycotoxins on growing crops can be markedly reduced by implementing balanced rotation systems and by choosing proper soil to plant tissues caused by birds, machinery and insects should be minimized, and weeds as well as plant diseases be controlled. Further management practices to reduce contamination include the prevention of drought stress by irrigation, the use of effective fungicides and the supply of nutrients to the plant in sufficient quantity and quality. In addition, the stage of maturity at harvest should not be overlooked.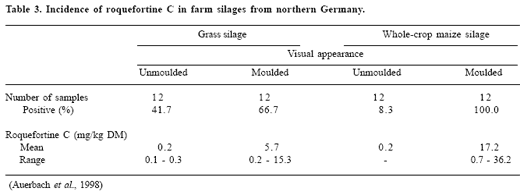
In order to prevent mould growth and mycotoxin formation during ensilage, it is critical to attain anaerobic conditions in the silo as soon as possible by rapid filling, good consolidation and perfect sealing. Air exclusion must be maintained by frequent checking and repair of the silo cover. Silages should be stored in gas-tight conditions for extended periods since the load of fungal spores declines with time (Auerbach, 1996), rendering the silage less prone to deterioration upon subsequent exposure to air (Philip and Spoelstra, 1997). Removal without loosening of the remaining silage mass during feedout will contribute to reduced risk of mould infestation and mycotoxin production in these feedstuffs. However, those technological measures are not always effective.
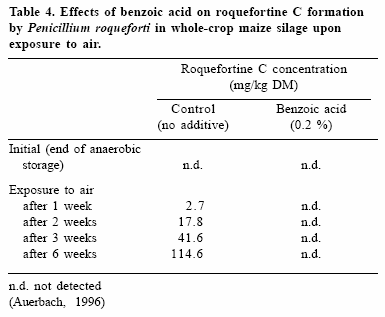
Several silage additives have been employed to control fungal growth in silages upon exposure to air (Weinberg and Muck, 1996; Oude-Elferink et al., 2002). Organic acids such as acetic, propionic, butyric, benzoic and sorbic acids and their salts are potent mould inhibitors (Woolford, 1975; Fellner et al., 2001; Danner et al., 2003). Sorbic and benzoic acids were shown to be particularly efficient in suppressing growth of the common toxinogenic silage fungus P. roqueforti (Auerbach, 1996). Studies by Auerbach et al. (2000) showed that P. roqueforti-induced aerobic deterioration of fermented crops could be avoided by the use of benzoic acid, thereby also inhibiting mycotoxin biosynthesis in whole-crop maize silage (Auerbach, 1996; Table 4).
Bacterial inoculants have been widely employed in silage production. Homofermentative lactic acid acteria (LAB) consistently improve fermentation quality, but may have no effect or even a detrimental effect on aerobic stability due to a higher production of lactic acid at the expense of antimycotic acetic acid (Muck, 1997; Danner et al., 2003). Specifically selected strains of heterofermentative LAB, e.g. L. buchneri, may be another tool in safeguarding aerobic stability of particularly susceptible silage types, such as maize and small grain silages. The conversion of lactic acid into acetic acid and other compounds increases shelf life, but can substantially increase DM losses during fermentation (Driehuis et al., 1996; Driehuis et al., 2001).
New approaches in the development of silage additives have led to the combined use of several groups of additives in order to achieve a high efficacy and a broad range of applications. The simultaneous use of homofermentative lactic acid bacteria and benzoic acid and sorbic acid respectively as well as of mixtures of homofermentative and heterofermentative lactic acid bacteria positively affect aerobic stability and may therefore successfully mitigate the risk of mould growth and mycotoxin formation in silages (Auerbach, 1996; Auerbach et al., 2000; Driehuis et al., 2001; Owen, 2002).
Conclusions
Moulds and mycotoxins are common contaminants of forage crops and silages made from them in many areas of the world. They pose a potential health hazard to domestic livestock. Changes in environmental conditions from pre-ensiling through fermentation result in the establishment of a characteristic mycoflora, mainly represented by Penicillium, Aspergillus and Monascus species. In addition to field-derived mycotoxins, the proliferation of these filamentous fungi upon subsequent exposure to air during feedout can result in further increase in the mycotoxin load of silages. Good management of the growing crop, the ensiling process and the unloading phase must be employed to minimize mould and mycotoxin contamination, and the use of antimycotic silage additives should be implemented as a strategic tool in silage making technology. Adhering to those principles will certainly reduce the risk of nutrient losses by fungal development and, even more importantly, will substantially counteract the impact of their toxic metabolites on animal health and performance. If prevention failed and silage mycotoxins are suspected causes for health disorders and poor performance in cattle, the use of adsorbents may be an attractive and very practical option to alleviate their effects. These products, e.g. yeast cell wall derived glucans, have been successfully shown to adsorb a variety of toxic metabolites, thereby reducing their bioavailability to animals (Halama, 1982; Diaz et al., 1999; Piva and Galvano, 1999; Ely et al., 2001).
Acknowledgement
The author is deeply indebted to Dr. Elisabeth Oldenburg, Institute of Grassland and Crop Sciences of the Federal Research Centre of Agriculture, Braunschweig, Germany and Prof. Friedrich Weissbach, former Head of the Institute of Grassland and Forage Research of the Federal Research Centre of Agriculture, Braunschweig, Germany, for kindly providing unpublished data.
References
Adler, A. 1993. Untersuchungen zur mikrobiellen Qualität von Silagen. In: BAL-Bericht über die österreichweite Silagetagung vom 13./14. Januar 1993. Bundesanstalt für alpenländische Landwirtschaft, Gumpenstein, Austria, pp. 45-53.
All-Hilli, A.L. and J.E. Smith. 1979. Influence of propionic acid on growth and aflatoxin production of Aspergillus flavus. FEMS Microbiol. Letters 6:367-370.
Amend, R. 1990. Anhäufung der Mykotoxine Patulin, Penicillinsäure, PR-Toxin und Mycophenolsäure durch Penicillien auf Maissilage und halbsynthetischem Medium. Ph.D. Thesis. University of Hohenheim, Germany.
Armbruster, G. 1994. Futtermittelhygienische Untersuchungen von Silagen und Vorkommen des Mykotoxins Roquefortin. Ph.D. Thesis. Ludwig-Maximilians-University, Munich, Germany.
Auerbach, H. 1996. Verfahrensgrundlagen zur Senkung des Risikos eines Befalls von Silagen mit Penicillium roqueforti und einer Kontamination mit Mykotoxinen dieses Schimmelpilzes. Landbauforsch. Völkenrode. Sonderheft 168:1-167.
Auerbach, H. and C. Geissler. 1992. Mykotoxine in Rauh- und Saftfuttermitteln für Wiederkäuer. Übers. Tierern. 20:167-208.
Auerbach, H., E. Oldenburg and F. Weissbach. 1998. Incidence of Penicillium roqueforti and roquefortine C in silages. J. Sci. Food Agric. 76:565-572.
Auerbach, H., E. Oldenburg and G. Pahlow. 2000. Prevention of Penicillium roqueforti-associated aerobic deterioration of maize silage by various additives. Mycotoxin Res. 16A (2):146-149.
Bauer, J. 2002. Mycotoxins in feedstuffs for ruminants: Biochemical effects and clinical relevance. In: Proceedings of the XXII World Buiatrics Congress (M. Kaske, H. Scholz and M. Höltershinken, eds). Hildesheimer Druck- u. Verlags-GmbH, Hildesheim, Germany, pp. 168-180.
Böhm, J., E. Razazzi, J. Grajewski and I. Styriak. 1999. Aktuelles und Zukunftsperspektiven der Biodegradation von Mykotoxinen. In: Proceedings 21. Mykotoxin-Workshop (H. Rosner and P. Kielstein, eds). 7-9 Juni 1999, Jena, Germany. pp. 188-191.
CAST. 1989. Council for Agricultural Science and Technology Task Force Report No. 116. Mycotoxins: Economic and health risks. Ames, IA, USA.
Chujian, L., K.K. Bolsen, B.E. Brent and D.Y.C. Fung. 1992. Epiphytic lactic acid bacteria succession during the pre-ensiling and ensiling periods of alfalfa and maize. J. Appl. Bacteriol. 73:375-387.
Clevström, G., T. Möller, B. Göransson, A. Liljenslöö and H. Ljunggren. 1989. Influence of formic acid on fungal flora of barley and on aflatoxin production in Aspergillus flavus Link. Mycopathol. 107:101-109.
Cole, R.J. 1976. Mycotoxins produced by Aspergillus fumigatus isolated from silage. International Symposium on Mycotoxins, Paris.
Cole, R.J. and R.H. Cox. 1981. Handbook of toxic fungal metabolites. Academic Press Inc., New York, USA.
Cole, R.J., J.W. Kirksey, J.W. Dorner, D.M. Wilson, J.C. Johnson, A.N. Johnson, D.M. Bedell, J.P. Springer, K.K. Chexal, J.C. Clardy and R.H. Cox. 1977. Mycotoxins produced by Aspergillus species isolated from moulded silage. J. Agric. Food Chem. 25:826-830.
Damaglou, A.P., W. Shannon and G.A. Downey. 1984. The interactions between Fusaria and their toxins in grass silage. J. Sci. Food Agric. 35:279-284.
Danner, H., M. Holzer, E. Mayrhuber and R. Braun. 2003. Acetic acid increases stability of silage under aerobic conditions. Appl. Environ. Microbiol. 69(1):562-567.
Diaz, G.J. and H.J. Boermans. 1994. Fumonisin toxicosis in domestic animals: A review. Vet. Human Toxicol. 36:548-555.
Diaz, D.E., W.M. Hagler Jr., B.A. Hopkins, J.A. Eve and L.W. Whitlow. 1999. The potential of dietary sequestering agents to reduce the transmission of dietary aflatoxin to milk of dairy cows and to bind aflatoxins in vitro. J. Dairy Sci. 82:838.
Dieckmann, M.A. and M.L. Green. 1992. Mycotoxins and reproduction in domestic livestock. J. Anim. Sci. 70:1615-1627.
Dirheimer, G. 1998. Recent advances in the genotoxicity of mycotoxins. Rev. Med. Vet. 149:605-616.
Driehuis, F., S.F. Spoelstra, S.C.J. Cole and R. Morgan. 1996. Improving aerobic stability by inoculation with Lactobacillus buchneri. In: Proceedings of the XIth International Silage Conference (D.I.H. Jones, R. Dewhurst, R. Merry and P.M. Haigh, eds). University of Wales, Aberystwyth, 8-11 September 1996, pp. 106-107.
Driehuis, F., S.J.W.H. Oude Elferink and P.G. Van Wikselaar. 2001. Fermentation characteristics and aerobic stability of grass silage inoculated with Lactobacillus buchneri, with or without homofermentative lactic acid bacteria. Grass Forage Sci. 56:330-341.
Dutkiewicz, J., S.A. Olenchock, W.G. Sorenson, V.F. Gerencser, J.J. May, D.S. Pratt and V.A. Robinson. 1989. Levels of bacteria, fungi and endotoxin in bulk and aerosolized corn silage. Appl. Environ. Microbiol. 55(5):1093-1099.
Dutton, M.F., K. Westlake and M.S. Anderson. 1984. The interaction between additives, yeasts and patulin production in silage. Mycopathol. 87:29-33.
El-Gazzar, F.E., G. Rusul and E.H. Marth. 1987. Growth and aflatoxin production by Aspergillus parasiticus NRRL 2999 in the presence of lactic acid and at initial different pH values. J. Food Prot. 50(11):940-944.
Ellis, W.O., J.P. Smith, B.K. Simpson and J.H. Oldham. 1991. Aflatoxins in food: Occurrence, biosynthesis, effects on organisms, detection and methods of control. Critical Rev. Food Sci. Nutr. 30 (3):403-439.
Ely, D.G., D.K. Aaron, S.E. Bar, V. Akay and J.A. Jackson. 2001. Continuing livestock production from endophyte-infected tall fescue and perennial ryegrass : recent studies with a toxin adsorbent. In: Nutritional biotechnology in the feed and food industries. Proceedings of Alltech´s Eighteenth Annual Symposium (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, Nottingham, UK, pp. 161-172.
Engel, G. and M. Teuber. 1978. Simple aid for the identification of Penicillium roqueforti Thom. European J. Appl. Microbiol. Biotechnol. 6:107-111.
Escoula, L. 1974. Moisissures toxinogenes des fourrages ensiles. I. Presence de patuline dans le fronts de coupe d´ensilages. Ann. Rech. Vet. 5:423-432.
Escoula, L. and G. Henry. 1975. Moisissure toxigenes des fourrages ensiles. V. Production d´acide byssochlamique en milieu liquide par Byssochlamys nivea Westling, Byssochlamys fulva Oliver et Smith et Paecilomyces varioti Bainier, isoles des ensilages. Ann. Rech. Veter. 59:537-543.
Escoula, L., J. Le Bars and G. Larrieu. 1972. Etudes sur la mycoflore des ensilages mycoflore des front de coupe d´ensilages de graminees fourrageres. Ann. Rech. Veter. 3:469-481.
Fellner, V., L.E. Phillip, S. Sebastian and E.S. Idziak. 2001. Effects of bacterial inoculant and propionic acid on preservation of high-moisture ear corn, and on rumen fermentation, digestion and growth
performance of beef cattle. Can. J. Anim. Sci. 81:273-280.
Fencl, Z. and J. Leopold. 1957. Mechanism of inhibition of by acetic acid of the germination of spores of Aspergillus niger. Nature 179:922.
Fenlon, D.R. and J. Wilson. 2000. Growth of Escherichia coli O157 in poorly fermented laboratory silages: a possible environmental dimension in the epidemiology of E. coli O157. Letters Appl. Microbiol. 30:118-121.
Frevel, H.-J., G. Engel and M. Teuber. 1985. Schimmelpilze in Silagen und Rohmilch. Milchwiss. 40(3):129-132.
Gedek, B., J. Bauer and H. Schreiber. 1981. Zur Mykotoxinbildung Silage-verderbender Schimmelpilze. Wien. Tierärztl. Mschr. 68:299-301.
Gotlieb, A. 1997. Causes of mycotoxins in silages. In: Proceedings from the Silage:Field to Feedbunk North American Conference. February 11-13 1997, Hershey, Pennsylvania. Northeast Regional Agricultural Engineering Service, Ithaca, New York, pp. 213-220.
Hacking, A. 1979. The effect of pH on the production of patulin by Paecilomyces in silage. In: Proceedings of a 3rd Meeting on Mycotoxins in Animal Disease, 1978 (G.A. Pepin, D.S.P. Patterson and B.J. Shreeve, eds). HMSO, Weybridge, Berkshire, UK.
Hacking, A. and W.R. Rosser. 1981. Patulin production by Paecilomyces species isolated from silage in the UK. J. Sci. Food Agric. 32:620-623.
Halama, A.K. 1982. Mykotoxikose bei Nutztieren und ihre Bekämpfung. Wien. tierärztl. Mschr. 69:109-314.
Inglis, G.D., L.J. Yanke, L.M. Kawchuk and T.A. McAllister. 1999. The influence of bacterial inoculants on the microbial ecology of aerobic spoilage of barley silage. Can. J. Microbiol. 45:77-87.
Jarvis, B., D.A.L. Seiler, A.J.L. Ould and A.P. Williams. 1983. Observations on the enumeration of moulds in foods and feedingstuffs. J. Appl. Bacteriol. 55:325-336.
Jonsson, A. and G. Pahlow. 1984. Systematic classification and biochemical characterization of yeasts growing in grass silage inoculated with Lactobacillus cultures. Anim. Res. Dev. 20:7-22.
Jonsson, A., H. Lindberg, S. Sundas, P. Lingvall and S. Lindgren. 1990. Effect of additives on the quality of big-bale silage. Anim. Feed Sci. Technol. 31:139-155.
Kämpfe, K. 1999. Ergebnisse von Mykotoxinuntersuchungen in Korngetreide und Futtermitteln. In: Proceedings 111. VDLUFA-Kongress in Halle/Saale. September 13-17 1999, Halle/Saale, Germany, pp. 313-315.
Kalac, P. and M.K. Woolford. 1982. A review of some aspects of possible associations between the feeding of silage and animal health. Br. Vet. J. 138:305-320.
Kiessling, K.-H. 1986. Biochemical mechanism of action of mycotoxins. Pure Appl. Chem. 58:327-338.
Lacey, J. 1989. Pre- and post-harvest ecology of fungi causing spoilage of foods and other stored products. J. Appl. Bacteriol. 67:Symposium Supplement 11S-25S.
Lacey, J. and N. Magan. 1991. Fungi in cereal grains: Their occurrence and water and temperature relationships. In: Cereal grain - Mycotoxins, fungi and quality in drying and storage. Developments in Food Science 26 (J. Chelkowski, ed). Elsevier, Amsterdam-London-New York-Tokyo, pp. 77-118.
Lepom, P., H. Baath and O. Knabe. 1988. Occurrence of Fusarium species and their mycotoxins in maize. 3. The influence of silaging on the zearalenone content in CCM maize. Arch. Anim. Nutr. 38:817-823.
Lepom, P., O. Knabe and H. Baath. 1990. Vorkommen von Fusarium-Arten und ihren Mykotoxinen auf Silomais.7. Mitteilung. Bildung von Deoxynivalenol (DON) in einem künstlich mit F. culmorum infizierten Silomaisbestand sowie der Einfluss der Silierung auf die Stabilität des gebildeten DON. Arch. Anim. Nutr. 40:1005-1012.
Lieu, F.Y. and L.B. Bullerman. 1978. Binding of patulin and penicillic acid to glutathione and cysteine and toxicity of the resulting adducts. Milchwiss. 33:16-20.
Lindenfelser, L.A. and A. Ciegler. 1970. Studies on aflatoxin detoxification in shelled corn by ensiling. J. Agric. Food. Chem. 18:640-643.
Lord, K.A., J. Lacey, J. Cayley and R. Manlove. 1981. Fatty acids as substrates and inhibitors of fungi from propionic acid treated hay. Trans. Br. Mycol. Soc. 77:41-45.
Lück, E. 1985. Chemische Lebensmittelkonservierung. Springer-Verlag, Berlin, Germany.
Marquardt, R.R. 1996. Effect of mould and their toxins on livestock performance: a western Canadian perspective. Anim. Feed Sci. Technol. 58:77-89.
Miller, J.D. 1995. Fungi and mycotoxins in grain: Implications for stored product research. J. stored Prod. Res. 31(1):1-16.
Mills, J.A. and L. Kung, Jr. 2002. The effect of delayed ensiling and application of a propionic acid-based additive on the fermentation of barley silage. J. Dairy Sci. 85:1969-1975.
Mor, S. and K. Singh. 2000. Incidence of toxigenic molds in dairy products and animal feeds. Indian J. Anim. Sci. 70(7):766-768.
Moreau, S. 1981. Le Penicillium roqueforti, morphologie, physiologie, interet en industrie fromagere, mycotoxines (Revision bibliographique). Lait LX:254-271.
Moss, M. 1991. Economic importance of mycotoxins-recent evidence. Internat. Biodeter. 27:195-204.
Muck, R. 1997. Effects of silage additives on ensiling. In: Proceedings from the Silage:Field to Feedbunk North American Conference. February 11-13 1997, Hershey, Pennsylvania. Northeast Regional Agricultural Engineering Service, Ithaca, New York, pp. 187-199.
Muck, R.E. and K.K. Bolden. 1991. Silage preservation and silage additive products. In: Field guide for hay and silage management in North America (K.K. Bolsen, J.E. Baylor and M.E. McCullough, eds). National Feed Ingredients Association, West Des Moines, IA, USA, pp. 105-126.
Müller, H.-M. 1983. A survey of methods of decontaminating mycotoxins. Anim. Res. Developm. 18:8-37.
Müller, H.-M. and G. Hörber. 1982. Development of fungi and decomposition of propionic acid in moistened grain corn. Zbl. Microbiol. 137:214-217.
Müller, H.-M. and R. Amend. 1997. Formation and disappearance of mycophenolic acid, patulin, penicillic acid and PR toxin in maize silage inoculated with Penicillium roqueforti. Arch. Anim. Nutr. 50:213-225.
Müller, H.-M., E. Schröppel and M. Thaler. 1981. Einfluss von Propionsäuredosierung und Kornfeuchte auf die Entwicklung und Mykotoxinbildung von Penicillien und Aspergillen in Körnermais. Landwirtsch. Forsch. 34(1-2):23-33.
Müller, H.-M., J. Reimann, U. Schumacher and K. Schwadorf. 1997. Fusarium toxins in wheat harvested during six years in an area of southwest Germany. Nat. Toxins 5:24-30.
Müller, M. 1991. Untersuchungen zum Alternaria-Befall von Silomais und Heu. Zentralbl. Mikrobiol. 146:481-488.
Müller, M. 1992. Toxin production of moulds of the genus Alternaria. Zentralbl. Mikrobiol. 147:207-213.
Nakane, M. 1946. On the moulds of ensilage. J. Appl. Mycol. 1:17-29; 30-41.
Niles, E.V. 1980. Fungi in stored barley treated with systemic fungicides. Pest Sci. 11:458-466.
Nibbelink, S.K. 1986. Aflatoxicosis in food animals: A clinical review. Iowa State University Veterinarian. 48:28-31.
Nout, M.J.R., H.M. Bouwmeester, H. Haaksma and H. van Dijk. 1993. Fungal growth in silages of sugar beet press pulp and maize. J. Agric. Sci. Cambridge. 121:323-326.
Ohomo, S. and H.K. Kitamoto. 1994. Detection of roquefortines in Penicillium roqueforti isolated from moulded maize silage. J. Sci. Food Agric. 64:211-215.
Oldenburg, E. 1991. Mycotoxins in conserved forages. Landbauforsch. Völkenrode. Sonderheft 123:191-205.
Oldenburg, E. 1996. In: Weissbach, F. and H. Auerbach: Wann ist der Mais siloreif? Mais 27 (2):72-77.
Oldenburg, E. 1999. Fungal secondary metabolites in forages: Occurrence, biological effects and prevention. Landbauforsch. Völkenrode. Sonderheft 206:91-109.
Oldenburg, E., H. Valenta and C. Sator. 2000. Risikoabschätzung und Vermeidungsstrategien bei der Lebensmittelerzeugung. Landbauforsch. Völkenrode. Sonderheft 216:5-34.
Olivigni, F.J. and L.B. Bullerman. 1977. Simultaneous production of penicillic acid and patulin by a Penicillium species isolated from cheddar cheese. J. Food Sci. 42:1654-1657, 1665.
Ominski, K.H., R.R. Marquardt, R.N. Sinha and D. Abramson. 1994. Ecological aspects of growth and mycotoxin production by storage fungi. In: Mycotoxins in grain:compounds other than aflatoxins (J.D. Miller and H.L. Trenholm, eds). Egan Press, St. Paul, MN, USA, pp. 287-314.
Oswald, I.P. and C. Comera. 1998. Immunotoxicity of mycotoxins. Rev. Med. Vet. 149:585-590.
Oude-Elferink, S., F. Driehuis, J. Gottschal and S. Spoelstra. 2002. Manipulating silage fermentation. Feed Mix 10(3):20-23.
Owen, T.R. 2002. The effects of a combination of a silage inoculant and a chemical preservative on the fermentation and aerobic stability of wholecrop cereal and maize silage. In: Conference Proceedings The XIIIth International Silage Conference (L.M. Gechie and C. Thomas, eds). September 11-13, 2002, Auchincruive, Scotland, pp. 196-197.
Parent-Massin, D. and R.E. Parchment. 1998. Haematotxicity of mycotoxins. Rev. Med. Vet. 149:591-598.
Parsons, D.J. 1991. Modelling gas flows in a silage clamp after opening. J. Agric. Engineering Res. 50:209-218.
Pelhate, J. 1975. Mycoflore des mais fourrages ensiles. Rev. Mycol. 39(2):65-95.
Pelhate, J. 1977. Maize silage: Incidence of moulds during conservation. Folia Vet. Lat. 7:1-16.
Petersson, S. and J. Schnürer. 1995. Biocontrol of mold growth in high-moisture wheat stored under airtight conditions by Pichia anomala, Pichia guiliermondii, and Saccharomyces cervisiae. Appl. Environ. Microbiol. 61(3):1027-1032.
Philip, S. and S.F. Spoelstra. 1997. Microorganisms and their use in treating animal feed and silage. Patent. International Publication Number WO 97/ 29644.
Pitt, R.E., R.E. Muck and N.B. Pickering. 1991. A model of aerobic fungal growth in silage. 2. Aerobic stability. Grass Forage Sci. 46:301-312.
Piva, A. and F. Galvano. 1999. Nutritional approaches to reduce the impact of mycotoxins. In: In: Biotechnology in the feed industry. Proceedings of the 15th Annual Symposium (T. P. Lyons and K. A. Jacques, eds). Nottingham University Press, Nottingham, UK, pp. 381-399.
Poisson, J. and B. Cahagnier. 1973. Probleme de la stabilisation de la microflore des grains humides par les acides organiques. Ann. Technol. Agric. 22(4):567-586.
Ramakrishna, N., J. Lacey and J.E. Smith. 1993. Effects of water activity and temperature on the growth of fungi interacting on barley grain. Mycol. Res. 97(11):1393-1402.
Ramos, A.J., F. Rull, V. Sanchis and S. Marin. 1998. Colonization of maize grain by Fusarium moniliforme and F. proliferatum in the presence of Aspergillus spp. and their impact on fumonisin production. Rev. Med. Vet. 149:523.
Richards, S.A. and D. Lloyd. 1966. Growth of Aspergillus glaucus on propionate. Biochem. J. 99:56.
Riley, R.T., K.A. Voss, W.P. Norred, R.P. Sharma, E. Wang and A.H. Merrill, Jr. 1998. Fumonisins: mechanism of mycotoxicity. Rev. Med. Vet. 149:617-626.
Rosiles, M.R. 1978. Study of aflatoxins in maize silage. Vet. Mexico 9:163-167.
Rust, S.R. and M.T. Yokoyama. 1992. Fermentation characteristics associated with aerobic instability of high-moisture corn. J. Prod. Agric. 5(4):454-457.
Schmerbauch, K.-J., E. Kaiser, C. Fürll and M. Schuster. 1999. Untersuchungen zur Einschränkung von Schimmelpilzwachstum in Grassilagen. In: Proceedings 111. VDLUFAKongress in Halle/Saale. September 13-17 1999, Halle/Saale, Germany, pp. 345-348.
Schneweis, I., K. Meyer, S. Hörmansdorfer and J. Bauer. 2000. Mycophenolic acid in silages. Appl. Environ. Microbiol. 66(8):3639-3641.
Schneweis, I., K. Meyer, S. Hörmansdorfer and J. Bauer. 2001. Metabolites of Monsacus ruber in silages. J. Anim. Nutr. Anim. Physiol. 85:38-44.
Scott, P.M. 1981. Toxins of Penicillium species used in cheese manufacture. J. Food Prot. 44(9):702-710.
Scott, P.M. 1988. Control of mycotoxins - an overview. In: Mycotoxins and Phycotoxins ´88. A collection of invited papers at the Seventh International IUPAC Symposium on Mycotoxins and Phycotoxins (S. Natori, K. Hashimoto and Y. Ueno, eds). 16-19 August 1988. Tokyo, Japan, Elsevier, Amsterdam, The Netherlands, pp. 127-136.
Scott, P.M. 2001. Analysis of agricultural commodities and foods for Alternaria mycotoxins. J. AOAC Internat. 84(6):1809-1817.
Scudamore, K.A. and C.T. Livesey. 1998. Occurrence and significance of mycotoxins in forage crops and silage: A review. J. Sci. Food Agric. 77:1-7.
Scudamore, K.A., M.T. Hetmanski, H.K. Chan and S. Collins. 1997. Occurrence of mycotoxins in raw ingredients used for animal feedingstuffs in the United Kingdom in 1992. Food Add. Contam. 14(2):157-173.
Scudamore, K.A., S. Nawaz and M.T. Hetmanski. 1998. Mycotoxins in ingredients of animal feeding stuffs. II. Determination of mycotoxins in maize and maize products. Food Add. Contam. 15(1):30-55.
Shier, W.T. 1998. Estrogenic mycotoxins. Rev. Med. Vet. 149:599-604.
Skaar, I. 1996. Mycological survey and characterization of the microbiota of big bale grass silage. Ph. D. Thesis, Norwegian College of Veterinary Medicine, Oslo, Norway.
Skaar, I. and H. Stenwig. 1996. Malt-yeast extractsucrose agar, a suitable medium for enumeration and isolation of fungi from silage. Appl. Environ. Microbiol. 62(10):3614-3619.
Smith, J.E. and G. Harran. 1993. Microbial degradation of mycotoxins. Internat. Biodeterior. Biodegrad. 32(1-3):205-211.
Smith, M.E., C.W. Lewis, J.G. Anderson and G.L. Solomons. 1994. A literature review carried out on behalf of the Agro-industrial division, E2, of the European Commission Directorate-General XII for scientific research and Development. Mycotoxins in Human Nutrition and Health.
Steyn, P.S. 1998. The biosynthesis of mycotoxins. Rev. Med. Vet. 149:469-478.
Sundberg, M. and P. Häggblom. 1999. Microbiological status in tower silos. In: Proceedings of the XIIth International Silage Conference (T. Pauly, ed). July 5-7 1999, Swedish University of Agricultural Sciences (SLU) Uppsala, Sweden, pp. 329-330.
Thylin, I. 2000. Methods of preventing growth of Clostridium tyrobutyricum and yeasts in silage. Acta Univerisitatis Agriculturae Sueciae. Agraria 223.
Trenholm, H.L., D.B. Prelusky, J.C. Young and J.D. Miller. 1989. A practical guide to the prevention of Fusarium mycotoxins in grain and animal feedstuffs. Arch. Environ. Contam. Toxicol. 18:443-451.
Trenholm, H.L., L.L. Charmley and D.B. Trenholm. 1997. Immune suppressing and other adverse effects of silage mycotoxins in dairy cattle. In: Proceedings of the C. F. I. A. Eastern Nutrition Conference 1997. May 6-7 1997, University of Guelph, Guelph, Ontario, pp. 21-27.
Tubaki, K. 1956. Penicillium isolates from toxic ensilage. Trans. Mycol. Soc. Japan 1:6-7.
Tüller, G., N. Hertkorn, W. Richter and J. Bauer. 1995. Aspergillus fumigatus in maize silage: Formation of fumitremorgen B and verruculogen. Landbauforsch. Völkenrode. Sonderheft 157:124-127.
Vesely, D., D. Vesela and A. Adamkova. 1981. Nalez plisne Penicillium roqueforti produkujici PR-Toxin v kukuricnych silazich. Veterinarni Medicina (Prague) 26:109-115.
Vivier, D., M. Rivemale, J.P. Reverbel, R. Ratomahenina and P. Galzy. 1992. Some observations on the physiology of Penicillium roqueforti Thom and Penicillium cyclopium Westling. Lait 72:277-283.
Wei, R.D., P.E. Still, E.B. Smalley, H.K. Schnoes and F.M. Strong. 1973. Isolation and partial characterization of a mycotoxin from Penicillium roqueforti. Appl. Microbiol. 25:111-114.
Weinberg, Z.G. and R.E. Muck. 1996. New trends and opportunities in the development and use of inoculants for silage. FEMS Microbiol. Rev. 19:53-68.
Whitlow, L.W. and W.M. Hagler Jr. 2002. Mycotoxins in feeds. Feedstuffs. July 10, pp. 68-77.
Woolford, M. 1975. Microbiological screening of the straight chain fatty acids (C1-C12) as potential silage additive. J. Sci. Food Agric. 26:219-228.
Woolford, M. 1990. The detrimental effects of air on silage. J. Appl. Microbiol. 68:101-116.






