In vitro for detoxifying mycotoxins in contaminated feedstuffs
In vitro and in vivo testing of adsorbents for detoxifying mycotoxins in contaminated feedstuffs
Mycotoxins are a group of structurally diverse secondary fungal metabolites that occur as contaminants of grain worldwide. Aspergillus, Fusarium, Penicillium, and Claviceps species of fungi are ubiquitous in nature and under ideal conditions often infect economically important crops and forages in the field, during storage, transportation and processing. The most important mycotoxins found in the United States are aflatoxin B1, fumonisin B1, ochratoxin A, vomitoxin, zearalenone and the ergot alkaloids.
Many of these mycotoxins can cause serious health problems in livestock and their presence in agricultural commodities may result in serious economic losses. Acute mycotoxicosis outbreaks do occur, but most mycotoxin-associated animal health problems are obscure chronic conditions related to reduced efficiency of production and increased susceptibility to infectious disease. Recognizing these potential problems, the grain industry, animal producers, overseas buyers and inspection agencies are promoting on-site testing using ELISA test kits for the various mycotoxins.
Contaminated grains can be readily identified and at low levels of contamination can be safely used in livestock and poultry feed. Grain and screenings containing higher levels of mycotoxin contamination are generally unsafe for animal consumption. The use of microbial inactivation, mold inhibitors, fermentation, physical separation, thermal inactivation, irradiation, ammoniation (Goldblatt, 1971; Park et al., 1984; CAST, 1989), ozone degradation (Mckenzie et al., 1997, 1998), and sequestering agents have been reported for the decontamination and remediation of these highly contaminated feedstuffs.
Unfortunately, most of these measures are costly, time-consuming, and only partially effective. At the present time, the most promising and practical approach has been the addition of adsorbents to contaminated feed to selectively bind the mycotoxin during the digestive process, allowing the mycotoxin to pass harmlessly through the animal. Research indicates that a number of adsorbents are capable of binding aflatoxin and reducing or preventing its toxic effects (Phillips et al., 1988; Harvey et al., 1989, 1991a, 1991b, 1993; Kubena et al., 1990b, 1991, 1993; Ledoux et al., 1999). Natural sorbents are generally recognized as safe (GRAS) for animal feeds at levels of 2 % or less by FDA.
The major advantages of adsorbents include low cost, safety and the ease with which they can be added to animal feeds. However, not all adsorbents are equally effective in protecting livestock against the toxic effects of aflatoxin and several adsorbents have been shown to impair nutrient utilization (Chung et al., 1990; Kubena et al., 1993; Scheideler, 1993).
Recently, Dale (1998) noted that many of the adsorbents on the market today have not been adequately tested for in vivo efficacy, but are used based on in vitro data only. However, as Scheideler (1993) and Dwyer et al. (1997) have suggested, in vitro tests may not always be a reliable indicator of ability to bind a mycotoxin.
Therefore, it is important that adsorbents be subjected to in vivo evaluation both with respect to efficacy and to determine if impaired nutrient utilization from diets occurs. The objective of this paper is to provide a model for in vitro and in vivo evaluation of the efficacy of adsorbents to ameliorate the toxic effects of mycotoxins.
In vitro testing for mycotoxin binding by adsorbents
A search of the peer-reviewed scientific literature reveals that only limited information is available on in vitro mycotoxin binding studies. Information is almost exclusively limited to aflatoxin binding (Masimango et al., 1978; Phillips et al., 1988;Ramos and Hernandez, 1996; Grant and Phillips, 1998), although in vitro binding studies for zearalenone (Lemke et al., 1998), ochratoxin A (Rotter et al., 1989) and ergotamine (Chestnut et al., 1992) have also been published. Phillips et al. (1988), using radiolabeled aflatoxin B1, were the first to document that a variety of aluminum oxide compounds, silicas and aluminosilicates would bind aflatoxin in aqueous solution.
The adsorbent with the strongest binding ability for aflatoxin B1 was a hydrated sodium calcium aluminosilicate (HSCAS). The chemisorption of aflatoxin to HSCAS involves the formation of a complex by the b-keto-lactone or bilactone system of aflatoxin with uncoordinated metal ions in HSCAS (Phillips et al., 1990; Sarr et al., 1990).
Commercialization of this product initiated a search to find other products that would bind aflatoxin as well as many of the other commonly occurringmycotoxins. Because of the expense and limited ability of laboratories to work with radiolabeled mycotoxins, a number of laboratories developed thin layer chromatography (TLC) and high performance liquid chromatography (HPLC) procedures to rapidly and inexpensively screen large numbers of samples for mycotoxin binding ability. These methods were developed to simply identify potential candidates for in vivo testing.
The simple screening procedure we use is described below. It is typical of in vitro procedures used in most laboratories involved in testing the ability of adsorbents to bind mycotoxins.
EXPERIMENTAL DESIGN
Mycotoxins are readily available from Sigma Chemical Company. Primary stock solutions of each mycotoxin (1,000 μg/ml) are prepared in methanol or acetonitrile. Mycotoxin test solutions (2 μg/ml) for adsorption studies are prepared in distilled water from the methanolic stock solutions.
Mycotoxin concentrations in the test solutions are not based on levels known to cause problems in livestock, but are based on 1) solubility of the mycotoxin in aqueous solution; 2) the relative ease of analysis by HPLC; and 3) cost of the mycotoxin.
Aliquots (10 ml) of the mycotoxin test solutions are added to screw cap test tubes along with 100 mg of adsorbent. In order to measure loss due to non-specific binding and to eliminate exogenous peaks, two controls are prepared by adding 10 ml of the mycotoxin test solution without the addition of adsorbent, and 10 ml distilled water plus 100 mg adsorbent to test tubes. Each sample and control is run in triplicate. The test tubes are placed on a rotator shaker for 1 hr at room temperature and centrifuged at 3,000 rpm for 10 minutes. A 2 ml aliquot of the aqueous supernatant is removed for mycotoxin analysis. The original mycotoxin test solution is used as the HPLC standard for each mycotoxin.
HPLC analyses are performed on a Perkin-Elmer Model 250 liquid chromatograph pump equipped with an Perkin Elmer ISS 200 autosampler, a Perkin-Elmer 8.3 cm C18 cartridge column (3 μm particle size). Detection is with a Perkin-Elmer LS-4 fluorescence spectrophotometer or a Perkin Elmer LC90UVdetector. Flowrate of mobile phase is 1 ml/min. The mobile phases and detection wavelengths used for each mycotoxin are presented in Table 1. Percent mycotoxin bound is calculated fromthe difference between the initial and final concentration of mycotoxin in the aqueous supernatant.
Table 1.HPLC mobile phase and detection wavelengths for mycotoxin analysis.

*F=fluorescence, Ex=excitation wavelength, Em=emission wavelength, UV=ultraviolet
In vitro mycotoxin concentrations of 2 μg/ml in aqueous test solutions were selected because many of the mycotoxins have only limited solubility in water. For example, the solubility of aflatoxin B1 in water is estimated to be 11-33 μg/ml by Grant and Phillips (1998), while the solubility of zearalenone in water is estimated to be less than 4 μg/ml by Lemke et al. (1998) at neutral pH and 37w C. Addition of higher levels of mycotoxins may result in precipitation and lead to erroneous adsorption results.
The simple in vitro procedure allows a large number of samples to be examined rapidly and inexpensively. The absence of sample transfers and filtration steps minimizes error due to nonspecific binding, etc. The procedure is used as a yes/no test to identify sorbents that strongly bind mycotoxins relative to sorbents that have little if any binding ability. A sorbent demonstrating strong binding ability (>80-90%) in distilled water can be further evaluated in vitro for mycotoxin binding ability over a wide range of mycotoxin and adsorbent concentrations, temperatures, pHs, and time periods (Ramos and Hernandez, 1996; Grant and Phillips, 1998; Lemke et al., 1998).
Additional in vitro information on surface adsorption of mycotoxins on binders can be obtained by using Langmuir and Freundlich adsorption isotherms (Ramos and Hernandez, 1996). Adsorption isotherms plot the amount of mycotoxin adsorbed per unit weight of adsorbent versus the mycotoxin concentration in solution at equilibrium and constant temperature (see Figure 1) and allow the determination of binding capacity and affinity of the sorbent for the compound adsorbed. Grant and Phillips (1998) were able to use multiple isotherm equations to determine not only capacity and affinity but also average capacity, enthalpy of binding, heterogeneity coefficient, multiple site distribution coefficients, and multisite capacity of HSCAS for aflatoxin B1.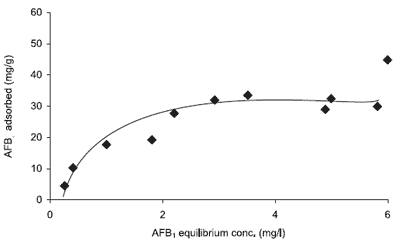
Figure 1. Isotherm plot of aflatoxin B1 adsorption to a sorbent.
Model for in vivo testing of adsorbents in poultry
EXPERIMENTAL DESIGN
Once an adsorbent is identified from in vitro testing as a potential candidate for in vivo testing (>80-90% binding), the following approach is then used to evaluate the adsorbent. An example of dietary treatments required to evaluate the efficacy of an adsorbent to ameliorate the toxic effects of a singlemycotoxin is presented in Table 2.
Diet 1 is a control diet that should contain no mycotoxin or adsorbent. The diet should be a typical cornsoybean meal starter diet used in the broiler industry and should be formulated to meet or exceed the nutritional requirements of growing chicks as recommended by the National Research Council (NRC, 1994).
In our laboratory, we usually formulate a basal diet that meets or exceeds NRC requirements and allows for the substitution of small quantities of the adsorbent and mycotoxin. The basal diet is then split into four portions and the adsorbent and clay are added to produce diets 2, 3, and 4. Diet 2 will contain the adsorbent to be tested and is included to provide information on the effects (negative or positive) of the adsorbent when fed alone.
This treatment is critical because some adsorbents have been shown to compromise the nutritional integrity of the diet (Chung et al., 1990;Kubena et al., 1993; Scheideler, 1993).
Diet 3 will contain the mycotoxin at a level that will ensure a measurable toxic response. It is essential to produce a measurable effect against which any reduction in toxicity due to the adsorbent can be measured. Diet 4 contains both the adsorbent and themycotoxin and this treatment is included to demonstrate the ability of the adsorbent to ameliorate the toxic effects of the mycotoxin.
Dietary mycotoxin concentrations are confirmed by analysis and all diets are screened (Rottinghaus et al., 1982, 1992) for the presence of othermycotoxins including aflatoxin, citrinin, T-2 toxin, vomitoxin, sterigmatocystin, zearalenone, the fumonisins, diacetoxyscirpenol, and ochratoxin A prior to the start of the experiment.
Table 2.Dietary treatments for evaluating efficacy of an adsorbent to reduce the toxic effects of a mycotoxin.
A minimum of five pen replicates of six day-old chicks should be assigned to each of the four dietary treatments for 21 days. Data are analyzed as a 2 × 2 factorial by analysis of variance using the General Linear Models procedure of SAS (SAS Institute, 1985). The means for treatments showing significant differences in the analysis of variance are then compared using the Fisher’s protected least significant difference procedure (Snedecor and Cochran, 1967).
LEVEL OF MYCOTOXIN AND ADSORBENT FOR IN VIVO TESTING
The level of mycotoxin to use will depend on the toxicity of the individual mycotoxin, and as indicated previously, on the level that will result in a measurable toxic effect. A list of mycotoxins and levels that have consistently been shown to cause a measurable toxic effect in broiler chicks is summarized in Table 3. T-2 toxin is recommended as being representative of the tricothecenes because of its greater toxicity compared with other tricothecenes.
The level of adsorbent to be used for testing will depend on two major considerations. First and most important is to test at a level that is being recommended for dietary inclusion. The second consideration is to include a level that represents the highest possible inclusion level. As indicated previously, most natural adsorbents are recognized as safe for animal feeds at levels of up to 2% by FDA. However, because some of the current adsorbents on the market may have been activated (chemically or physically treated) they may not be harmless at levels up to 2% of the diet.
Therefore one approach is to test at least two levels, the recommended level and the highest possible inclusion level. Testing of the highest inclusion level serves as an additional demonstration of the safety of the product.
SELECTION OF RESPONSE VARIABLES
The obvious and most important response variable of interest to producers is performance. However, as Dale (1998) correctly points out, evaluation of effects on performance (feed intake, body weight gain, feed conversion) is probably not adequate for satisfactory evaluation.
Using aflatoxin as an example, Dale (1998) recommends that such sensitive parameters as serum proteins and carotenoids be evaluated, and that effects on immune response should also be measured. We use a number of response variables including performance, organ weights, serum chemistry, gross pathology, and histopathology. We have also recently developed and tested a panel of immune assays that will be used in the future to further evaluate mycotoxins (Li, 1998).
With respect to gross pathology, all birds are examined for signs of pathology due either to themycotoxin or resulting from nutritional deficiencies that may have been caused by addition of the adsorbent. Brains are examined for the lesions of cerebellar swelling, edema, and hemorrhage as seen with vitamin E deficiency (Austic and Scott, 1997).
The mucosal surface of the crop and esophagus are examined for the multiple white foci, indicative of squamous metaplasia of the subepithelialmucous glands, observed with vitamin A deficiency (Austic and Scott, 1997). The proximal tibial growth plate is examined for widening of the zones of proliferation and/or hypertrophy as seen in cases of vitamin D, calcium and/or phosphorus deficiency (Austic and Scott, 1997). The tibia is also broken as a simple method to evaluate bone strength.
The skeletal muscles and abdominal cavity are examined for the hemorrhages observed in cases of vitamin K deficiency (Austic and Scott, 1997). The legs are also examined for bowing and/or perosis as can occur with deficiencies in nicotinic acid, choline, and/or manganese (Austic and Scott, 1997). The skin and feathers of the chicks are also examined for indications of pantothenic acid and biotin deficiency (Austic and Scott, 1997). With respect to histopathology, only the target organs of the testmycotoxin are microscopically evaluated.
Table 3 contains a list of mycotoxins and their effects on various response variables in broiler chicks. Using this table it is relatively easy to select the appropriate response variables. For example, if evaluating aflatoxin, response variables should include performance, serum proteins, liver and kidney weights, and microscopic evaluation of liver and kidney for pathologic changes.
Table 3. Toxic effects of selected mycotoxins in broiler chicks.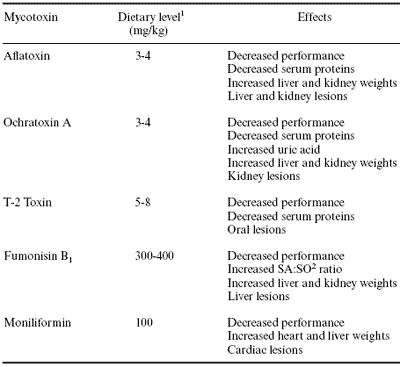
1Range reported to consistently cause toxic effects listed.
2SA:SO = sphinganine to sphingosine ratio.
Efficacy of adsorbents to bind mycotoxins
Since it was first demonstrated (Phillips et al., 1988, 1990) that a specific hydrated sodium calcium aluminosilicate (HSCAS) was effective in ameliorating the toxic effects of aflatoxin in vivo, a large number of other adsorbents have been tested for efficacy against aflatoxin. Results indicate that there is great variability in the efficacy of these compounds in vivo; and in many cases in vitro efficacy is not related to in vivo efficacy.
Recently, there has been a tremendous increase in the number of these adsorbents available commercially. In addition, claims are being made that these products are not only effective against aflatoxin but are also effective against a number of other mycotoxins including T-2 toxin, ochratoxin A, fumonisin B1, and deoxynivalenol (vomitoxin).
Unfortunately, most of these claims are based on in vitro evaluation, which as we have discussed previously is not always correlated with in vivo efficacy. To date, results of well-designed in vivo studies published in refereed journals indicate that none of the adsorbents tested provided any protection against T-2 toxin, ochratoxin A, cyclopiazonic acid, diacetoxyscirpenol, or fumonisin B1 (Kubena et al., 1990a, 1992, 1993, 1998; Brown et al., 1992; Huff et al., 1992; Dwyer et al., 1997; Bailey et al., 1998). In vivo studies conducted in our laboratory (Ledoux and Rottinghaus, unpublished) also indicate that none of adsorbents tested thus far have afforded protection against fumonisin B1, moniliformin, ochratoxin A or vomitoxin.
A review of the hypothesized mechanism for the efficacy of the HSCAS against aflatoxin may well explain the lack of efficacy of these adsorbents against other mycotoxins. As indicated earlier, the chemisorption of aflatoxin to HSCAS involves the formation of a complex by the ß-ketolactone or bilactone system of aflatoxin with uncoordinated metal ions in HSCAS (Phillips et al., 1990; Sarr et al., 1990).
A review of the chemical structures of the other mycotoxins indicates, with the possible exception of ergot alkaloids, that none of them have a similar ß-keto-lactone or bilactone system that is thought to be essential for chemisorption to HSCAS to occur. Therefore, unless these mycotoxins have some other type of structural system that would allow chemisorption to HSCAS to occur, it is not surprising that HSCAS adsorbents are not effective in reducing or preventing the toxic effects of these mycotoxins.
| Conclusion In conclusion, the in vitro and in vivo models outlined in this paper will provide the livestock industry with the means to critically evaluate adsorbents currently available. Additionally, these procedures can be used to develop and evaluate the efficacy of new products to alleviate the toxic effects of mycotoxins. |
References
Austic, R.E. and M.L. Scott. 1997. Nutritional diseases. In: Diseases of Poultry, 10th ed. (B.W. Calnek, H.J. Barnes, C.W. Beard, L.R. McDougald, and Y.M. Saif eds.), Iowa State University Press, Ames, Iowa. pp. 47-73.
Bailey, R.H., L.F. Kubena, R.B. Harvey, S.A. Buckley and G.E. Rottinghaus. 1998. Efficacy of various inorganic sorbents to reduce the toxicity of aflatoxin and T-2 toxin in broiler chickens. Poultry Sci. 77:1623-1630.
Brown, T.P., G.E. Rottinghaus and M.E. Williams. 1992. Fumonisin mycotoxicosis in broilers: Performance and pathology. Avian Diseases 36:450-454.
Council for Agricultural Science and Technology Task Force Report #16. 1989. Mycotoxins:Economic and Health Risks. Ames, IA.
Chestnut, A.B., P.D. Anderson, M.A. Cochran, H.A. Fribourg and K.D. Gwinn. 1992. Effects of hydrated sodium calcium aluminosilicate on fescue toxicosis and mineral absorption. J. Anim. Sci. 70:2838-2846.
Chung, T.K., J.W. Erdman, Jr. and D.H. Baker. 1990. Hydrated sodium calcium aluminosilicate: effects on zinc, manganese, vitamin A, and riboflavin utilization. Poultry Sci. 69:1364-1370.
Dale, N. 1998. Mycotoxin binders: It’s time for real science. Poultry Digest 57:38-39.
Dwyer, M.R., L.F.Kubena, R.B. Harvey,K.Mayura, A.B. Sarr, S. Buckley, R.H. Bailey and T. D. Phillips. 1997. Effects of inorganic adsorbents and cyclopiazonic acid in broiler chicks. Poultry Sci. 76:1141-1149.
Goldblatt, L.A. 1971. Control and removal of aflatoxin. J. Am. Oil Chem. Soc. 48:605-610.
Grant, P.G. and T.D. Phillips. 1998. Isothermal adsorption of aflatoxin B1 on HSCAS clay. J. Agric. Food Chem. 46:599-605.
Harvey, R.B., L.F. Kubena, T.D. Phillips, W.E. Huff and D.E. Corrier. 1989. Prevention of aflatoxicosis by addition of hydrated sodium calcium aluminosilicate to the diets of growing barrows. Am. J. Vet. Res. 50:416-420.
Harvey, R.B., L.F. Kubena, T.D. Phillips,M.E. Elissalde andW.E. Huff. 1991a. Diminution of aflatoxin toxicity to growing lambs by dietary supplementation with hydrated sodium calcium aluminosilicate. Am. J. Vet. Res. 52:152-156.
Harvey, R.B., T.D. Phillips, J.A. Ellis, L.F. Kubena,W.E. Huff and H.D. Petersen. 1991b. Effects on aflatoxin M1 residues in milk by addition of hydrated sodium calcium aluminosilicate to aflatoxin-contaminated diets of dairy cows. Am. J.Vet. Res. 52:1556-1559.
Harvey, R.B., L.F.Kubena, M.H. Elissalde and T.D. Phillips. 1993. Efficacy of zeolitic ore compounds on the toxicity of aflatoxin to growing broiler chickens. Avian Dis. 37:67-73.
Huff, W.E., L.F. Kubena, R.B. Harvey and T.D. Phillips. 1992. Efficacy of hydrated sodium calcium aluminosilicate to reduce the individual and combined toxicity of aflatoxin and ochratoxin A. Poultry Sci. 71:64-69.
Kubena, L.F., R.B. Harvey, W.E. Huff, D.E. Corrier, T.D. Phillips and G.E. Rottinghaus. 1990a. Efficacy of a hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and T-2 toxin. Poultry Sci. 69:1078-1086.
Kubena, L.F., R.B. Harvey, T.D. Phillips, D.E. Corrier and W.E. Huff. 1990b. Diminution of aflatoxicosis in growing chickens by the dietary addition of hydrated sodium calcium alumiunosilicate. Poultry Sci. 69:727-735.
Kubena, L.F.,W.E. Huff, R.B. Harvey, A.G. Yersin, M.H. Elissalde, D.A. Witzel, L.E. Giroir, T.D. Phillips and H.D. Petersen. 1991. Effects of a hydrated sodium calcium aluminosilicate on growing turkey poults during aflatoxicosis. Poultry Sci. 70:1823-1830.
Kubena, L.F., R.B. Harvey, T.D. Phillips and B.A. Clement. 1992. Effects of several sorbent materials onmycotoxicosis in broiler chickens. Poultry Sci. 71(Suppl. 1):161.
Kubena, L.F., R.B. Harvey,W.E. Huff, M.H. Elissalde, A.G. Yersin, T.D. Phillips and G.E. Rottinghaus. 1993. Efficacy of a hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and diacetoxyscirpenol. Poultry Sci. 72:51-59.
Kubena, L.F., R.B. Harvey, R.H. Bailey, S.A. Buckley and G.E. Rottinghaus. 1998. Effects of a hydrated sodium calcium aluminosilicate (T-Bind) on mycotoxicosis in young broiler chickens. Poultry Sci. 77:1502-1509.
Ledoux, D.R., G.E. Rottinghaus, A.J. Bermudez and M. Alonso- Debolt. 1999. Efficacy of a hydrated sodium calcium aluminosilicate to ameliorate the toxic effects of aflatoxin in broiler chicks. Poultry Sci. 78:204-210.
Lemke, S.L., P.G. Grant and T.D. Phillips. 1998. Adsorption of zearalenone by organophilic montmorillonite clay. J. Agric. Food Chem. 46:3789-3796.
Li, Y.C. 1998. Effects of Fusarium mycotoxins, fumonisin B1 and moniliformin, on selected immune responses in broiler chicks and turkey poults. Doctoral Dissertation, University of Missouri, Columbia, Missouri.
Masimango, N., J. Remacle and J.L. Ramaut. 1978. The role of adsorption in the elimination of aflatoxin B1 from contaminated media. Europ. J. Appl. Microbiol. 6:101-105.
McKenzie, K.S., A.B. Sarr, K. Mayura, R.H. Bailey, D.R. Miller, T.D. Rogers,W.P. Norred, K.A. Voss, R.D. Platner, L.F. Kubena and T.D. Phillips. 1997. Oxidative degradation and detoxification of mycotoxins using a novel source of ozone. Food and Chem. Toxicol. 35:807-820.
McKenzie, K.S., L.F. Kubena, A.J. Denvir, T.D. Rogers, G.D. Hitchens, R.H. Bailey, R.B. Harvey, S.A. Buckley and T.D. Phillips. 1998. Aflatoxicosis in turkey poults is prevented by treatment of naturallycontaminated corn with an ozone generated by electrolysis. Poultry Sci. 77:1094-1102.
National Research Council. 1994. Nutrient Requirements of Poultry, 9th ed. National Academy of Science, Washington, D.C.
Park, D.L., L.S. Lee and S.A. Kolton. 1984. Distribution of ammoniarelated aflatoxin reaction products in cottonseed meal. J. Am. Oil Chem. Soc. 61:1071-1074.
Phillips, T.D., L.F. Kubena, R.B. Harvey, D.R. Taylor and N.D. Heidelbaugh. 1988. Hydrated sodium calcium aluminosilicate: A high affinity sorbent for aflatoxin. Poultry Sci. 67:243-247.
Phillips, T.D., A.B. Sarr, B.A. Clement, L.F. Kubena and R.B. Harvey. 1990. Prevention of aflatoxicosis in farm animals via selective chemisorption of aflatoxin. In:G.A. Bray andD.H.Ryan, ed. Pennington Center Nutrition Series Volume 1, Mycotoxins, Cancer, and Health, Louisiana State Univ. Press, Baton Rouge, LA. pp. 223-237.
Ramos,A.J. and E. Hernandez. 1996. In vitro aflatoxin adsorption by means of a montmorillonite silicate. A study of adsorption isotherms. Animal Feed Sci. Technology 62:263-269.
Rotter,R.G., A.A. Frohlich andR.R.Marquardt. 1989. Influence of dietary charcoal on ochratoxin A toxicity in Leghorn chicks. Can. J. Vet. Res. 53:489-453.
Rottinghaus, G.E., B. Olsen and G.D. Osweiler. 1982. Rapid screening method for aflatoxin B1, zearalenone, ochratoxin A, T-2 toxin, diacetoxyscirpenol and vomitoxin. In: Proceedings of the 25th Annual American Association of Veterinary Laboratory Diagnosticians, Nashville, TN. Pages 477-484
Rottinghaus, G.E., C.E. Coatney and H.C. Minor. 1992. A rapid sensitive thin layer chromatography procedure for the detection of fumonisin B1 and B2. J. Vet. Diagn. Invest. 4:326-329.
Sarr, A.B., B.A. Clement and T.D. Phillips. 1990. Effects of molecular structure on the chemisorption of aflatoxin B1 and related compounds by hydrated sodium calcium aluminosilicate. The Toxicologist 10(1):163.
SAS Institute. 1985. SAS User’s Guide: Statistics. Version 6 Edition. SAS Institute Inc. Cary, NC.
Scheideler, S. E. 1993. Effects of various types of aluminosilicates and aflatoxin B1 on aflatoxin toxicity, chick performance, and mineral status. Poultry Sci. 72:282-288.
Snedecor, G.W. andW.E. Cochran. 1967. Statistical Methods. 6th ed. The Iowa State University Press, Ames, IA.
Authors: DAVID R. LEDOUX1 and GEORGE E. ROTTINGHAUS2
Fusarium/Poultry Research Laboratory,
1 Department of Animal Science and
2 Veterinary Medical Diagnostic Laboratory, College of Veterinary Medicine, University of Missouri, Columbia, Missouri, USA







