Introduction
Dissemination is the process through which diseases increase both on a temporal and on a spatial scale. Dissemination involves three basic sub-processes: release, dispersion and deposit of pathogens. Release is defined as the removal of the pathogen from the location where it was produced; dispersion is the pathogen transport after its release; and deposit, in turn, is the settlement of the pathogen on a given surface. All pathogens produce a large number of propagules, which is why many are lost before reaching the host. Depending on the surface where a pathogen is deposited, its spread may or may not be successful. In the evolution between a pathogen and its host, different survival strategies can be developed. Strategies, such as hypha and spore survival in vector insects, ensure the initiation of the primary cycle of a disease at each new crop cycle (HIRST & SCHEIN, 1965; DOUGLAS & BEARD, 1996).
Insect vectors disseminate pathogens by carrying spores and hyphae during the host crop cycle. In some cases, in the absence of a host, these vectors retain the pathogens, contributing to their survival. When the insect vectors are not able to retain the pathogen for a prolonged period of time, it cannot be considered their action as a form of inoculum survival, leaving them only the role of disseminating agents. In some cases, insects can be considered biological vectors, in which spores can multiply and undergo cycle phases inside the insect’s organism, in these cases, the microorganism may reproduce and undergo cyclic changes inside the vector’s organism or the microorganism may only reproduce but not undergo cyclic changes inside the insect vector (HIRST & SCHEIN, 1965; HARWOOD & JAMES, 1979).
Red Root Rot or Sudden Death Syndrome SDS is caused by species of the Fusarium complex, either isolated or as a group. The absence of effective management techniques may complicate SDS control and the use of resistant cultivars can have a positive long-term result. Fusarium can remain in the soil for long periods of time in the chlamydo spores form even without the presence of the host plant. Symptoms of SDS begin with the yellowing of older leaves, causing damping off. SDS is most commonly disseminated through infected seeds and seedlings, carried by irrigation and rainfall water, agricultural workers and supplements (ZAMBOLIM & VALE, 2000).
However, studies have shown that insects are also important vectors, presenting adaptations for dispersing and inoculating Fusarium species, such as: Xylosandrus compactus (Eichhoff) (LE PELLEY, 1968); Dendroctonus sp. (WHITNEY, 1982); Reticulitermes flavipes (Kollar) (ZOBERI & GRACE, 1990); Diabrotica speciosa (Germar) (BERCELLINI & MALACALZA, 1994; SILVAWERNECK et al., 1995); Spodoptera litorallis (Boisduval) (ISMAIL & ABDEL-SATER, 1993); Xyleborus fornicatus (Eichh.) (KUMAR et al., 1998); Triatoma sp. (MORALES-RAMOS et al., 2000) and Hypothenemus hampei (Ferrari) (MORALES RAMOS et al., 2000; GAMA et al., 2006).
Considering the increase of white grubs (Coleoptera: Melolonthidae) and the lack of information related to the geographical distribution of root phytopathogens and soil insects as dispersers and/ or inoculators, this study aims to identify and evaluate qualitatively and quantitatively the dispersion of root phytopathogen inoculi in soy crops.
Materials and methods
Experiments were carried out in the district of Arroio Grande, Santa Maria, Rio Grande do Sul, Brazil (29º40’S and 53º44’W), both in January/ February 2012 and March/April/May 2013. In two soybean fields, trenches (50cm long x 25cm wide x 30cm deep), 20 samples (25m apart), were made to assess mycobiota associated with Melolonthidae larvae, with evaluations of the mouthparts, prothorax, cuticle and digestive tract. Seeds and roots of soybeans of RR Power BMX® cultivars were also collected.
The removed soil layer was sieved in a metal wire cloth of 2mm (0.80 x 0.40m). Soil samples were analyzed at the soil laboratory of the Center of Rural Sciences (CCR/UFSM) for: percentage of Clay, pH (water), percentage of organic matter, quantities of P, K, Ca, Mg, Al, Zn, B and Cu. Analysis of meteorological factors (INMET) was carried out using sample points: Temperature (°C), Humidity (%), Dew Point (%), Pressure (hPa),Wind speed (m/s), Radiation (kJ m-2), and Rain (mm).
In the laboratory of phytopathology at the UFSM, Melolonthidae larvae were superficially disinfected and rinsed in sterile distilled water, dissected and separated in parts: month parts, prothorax, cuticule, and digestive tract (alimentary canal) which were then placed in the eppendorf tubes containing 100mL of 0.85% saline solution (GAMA et al., 2006). Afterwards, larvae parts in saline solution were added to petri dishes with PDA (Potato Dextrose Agar) media and incubated in a growth chamber at 25.6ºC and photoperiod of 12h for seven days (SALGADO-NETO et al., 2015). Extraction of the isolated DNA was performed according to the method employing CTAB reagent (Cetyltrimethylammonium Bromide) described by DOYLE & DOYLE (1987).
The extracted genomic DNA was subjected to polymerase chain reaction (PCR) for amplification of the ITS region (Internal Transcribed Spacer) located between the genes encoding the 18S and 28S ribosomal RNAs. The primers used in the ITS region were ITS1 (5 ‘- TCCGTAGGTGAACCTGCGG - 3’) and ITS4 (5 ‘- TCCTCCGCTTATTGATATGC - 3’) (WHITE et al., 1990). Amplified products were purified by precipitation with polyethylene glycol (SCHMITZ & RIESNER, 2006), sequenced by the chain termination reaction method employing the reagent Big Dye 3.1 (Applied Biosystems) and analyzed by an automated capillary sequencer 3500L (Applied Biosystems). The sequences obtained were compared to those deposited in the GenBank at the National Center for Biotechnology Information (NCBI), using the Blastn program (ALTSCHUL et al., 1990). Identification was based on similarity to sequences present in GenBank type species.
The pathogenicity test was performed in plastic pots, using two seeds per pot of soybean cultivar RR POWER BMX®. PLANTMAX HA® substrate was used and pots were placed in a greenhouse under a natural photoperiod at ±30°C for 4 months. Each treatment was performed with 8 replications (pots) with two plants each. The 3 treatments (isolates of Fusarium spp. coming from the digestive tract of Melolonthidae larvae previously identified and an uninoculated control treatment) were arranged in a completely randomized design, forming 24 plots.
The severity of foliar symptoms was assessed using the scale based on the Huang-Hartman model (HUANG & HARTMAN, 1998). To evaluate the severity of root rot and colon damage, the roots were washed in running water to remove the substrate and evaluated using a scale based on the Achenbach model (ACHENBACH et al., 1996). After severity assessment, the roots were wrapped in paper bags, dried at 50°C for 24 hours and weighed to determine the dry weight of the root.
Data underwent one-factor non-parametric Kruskal-Wallis analysis to compare the means of the three treatments, at a 5% probability significance level (SIEGEL & CASTELLAN, 2008). When the test was significant (P<0.05), means were compared by the Dunn test, also at a 5% significance level. Data for the presence of Fusarium root and for seed germination were analyzed using the Exact Binomial test (at a 5% probability level), for comparing two by two treatments at a 95% confidence interval. Tests were performed using R Development Core Team software package (2014).
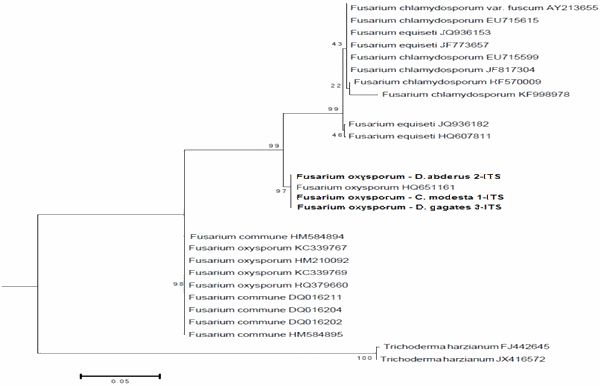
A phylogenetic dendrogram (Figure 1) was built for the isolates of Fusarium oxysporum Schlechtendal emend. Snyder & Hansen coming from the digestive tract (alimentary canal) of larvae of Cyclocephala modesta Burm, 1855, Dyscinetus gagates Burm, 1847 and Diloboderus abderus Sturm, 1826. Evolutionary distances were calculated using the Tamura-Nei model. Number of branches represents the number of bootstraps.
Figure 1 - Phylogenetic dendrogram built for the isolates of Fusarium oxysporum from the alimentary canal of Cyclocephala modesta, Dyscinetus gagates and Diloboderus abderus larvae, based on the statistical method “Neighbor-joining”, derived from ITSrDNA regions. The evolutionary distances were calculated using the Tamura-Nei model. The number of branches represents the “bootstrap” number.
The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura-Nei model (TAMURA & NEI, 1993). Percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically as follows. When the number of common sites was <100 or less than one fourth of the total number of sites, the maximum parsimony method was used; otherwise B.I.O.N.J. (Bio Informatics Neighbor Joining) method with M.C.L. (Maximum Composite Likelihood) distance matrix was used. Evolutionary analyses were conducted in MEGA5 (TAMURA et al., 2011).
The vectoring test with Melolonthidae larvae was conducted in the Integrated Pest Management Lab (LAB-MIP) at UFSM. Vectoring involves transportation, procurement and deposition of inoculum influenced by several factors, mainly by different techniques of spraying Trichoderma inoculum powder formulations, where the size of the product particles is a key factor. C. modesta and D. gagates larvae, 2nd and 3rd instars, collected from crops were placed individually in plastic cups containing autoclaved soil supplemented with carrot pieces (PARDO-LOCARNO et al., 2005) and Trichoderma powder formulation (5-15 microns) was sprayed directly onto the soil (SUTTON & PENG, 1993).
Results and discussions
Specific identification was made possible by extraction and DNA sequencing of the fungus inside the Melolonthidae larvae. Sequenced ITS1-5.8S-ITS4 regions of the ribosomal DNA showed presence of F. oxysporum. GenBank accession number KR082315.
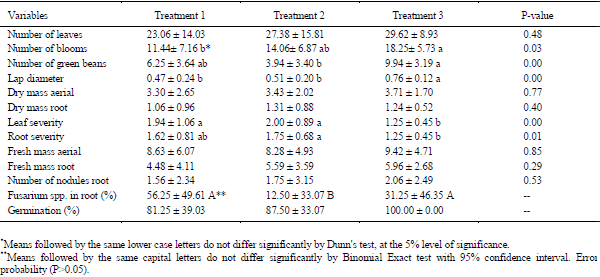
In the pathogenicity test (Table 1) in the soybean cultivar, RR Power BMX®, plants inoculated with F. oxysporum isolates originating from the gastric cecum (digestive tract) of the Melolonthidae larvae caused the following symptoms of SDS: internerval chlorosis of leaves, internerval necrosis and damping-off in seedlings and necrosis and death in shoots and branches. The frequency of F. oxysporum present in soybean roots (average 56.25%±3.55% standard error) also caused a reduction in germination (average 81.25%±2.20% standard error), higher leaf severity (average 1.94%±0.27% standard error), greater root severity (average 1.62%±0.23% standard error), and decreased lap diameter (average 0.47%±0,07% standard error).
Table 1 - Values of Kruskal-Wallis test in soybean RR power BMX for variable responses to the pathogenicity test in isolates of Fusarium oxysporum (T1), Fusarium incarnatum equiseti and Fusarium commune (T2) from the alimentary canal of Melolonthidae larvae and Cycloneda sanguinea. 2013/14 - Santa Maria/RS.
Spores and hyphae of the fungus Phallales (Phallus sp.) have been shown to serve as a source of protein and sugar to Trigona bees (BURR et al., 1996). In another study, Muscidae and Drosophillidae feed on Phallales fungi (Dictyophora indusiata and D. duplicata), eliminating intact spores with high germination rates (TUNO, 1998). Another study also showed that bees (Trigona crassipes and T. fulviventris) did not digest spores, indicating possible dispersion of intact spores eliminated in the feces (OLIVEIRA & MORATO, 2000).
Spores and hyphae of F. oxysporum present two formats. The macroconidia format is of short to medium length, straight to slightly curved relatively slender and thin-walled. The apical cell is tapered and curved, sometimes with a sling hook. The basal cell is foot shaped to pointed and is usually 3-septate. The microconidia format is oval, elliptical or kidney-shaped and usually non-septate, producing conidia in short monophialides, in false heads on the abundant aerial mycelia and chlamydo spores, singly or in pairs, but also can be reported in clusters or in short chains (NELSON et al., 1983; VENTURA, 2000, LESLIE & SUMMERELL, 2006). The sexual phase or teleomorph stage is unknown (LESLIE & SUMMERELL, 2006) and this is possibly due to the fact that it occurs inside the gastric cecum on the smooth or rough wall.
Spores and hyphae of fungi in some Coleoptera may be housed in mycetocytes, specialized cells of the intestinal epithelium, which can host symbiotic fungi. The distribution of mycetocytes varies, being located along the digestive tube or concentrated in certain regions of it. Symbiotic microorganisms (fungi) are released when the larval gut is destroyed during its metamorphosis, causing the loss of symbiotic associations. However, many microorganisms can survive in female insects (absent in adult males) and are incorporated in the later evagination structures, such as the bulb head, and in the end region of the ovipositor, as occurs in Anobiidae and Chrysomelidae. Microorganisms (fungi) have also been found in the gastric cecum larvae of four Coleoptera families such as, Anobiidae, Cerambycidae, Curculionidae and Chrysomelidae (DOUGLAS & BEARD, 1996).
The presence of spores and hyphae of F. oxysporum in the digestive tract and mouth parts of Melolonthidae larvae as well as in roots and stems of soybean observed in our study corroborates the notion of symbiotic relationships raised by MORALESRAMOS et al. (2000) in the association between Fusarium solani and H. hampei. The interaction of F. solani is important for feeding the larvae and adults of H. hampei, which in turn serve to spread the inoculi of F. solani to other host plants. In a study on larvae galleries in coffee berries, associations between Fusarium spp. and Trichoderma spp. were found with abundances of 33.3% and 18.5% respectively. The authors indicated the possibility that dispersion and inoculation of fungi by beetles occurred through boring because Trichoderma spp. was isolated exclusively from the mouth parts of H. hampei (GAMA et al., 2006).
The presence of spores and hyphae of F. oxysporum in mouth parts and digestive tract of Melolonthidae larvae and in galleries in the soybean roots made by the larvae, reported in the pathogenicity test, indicates a close interaction between Melolonthidae larvae and F. oxysporum directly related to symptoms of SDS. The sexual phase or teleomorph stage of F. oxysporum is probably unknown because it occurs inside the digestive tract (gastric cecum) of Melolonthidae larvae. Its characteristics, such as macroconidia and microconidia formats, the presence or absence of chlamydo spores, branched or not conidia and presence of monophialides or polyphialides, which are taken into account for the identification of species and subspecies (formae specialis) are likely due to adaptations necessary for the dissemination, dispersion and deposition (inoculation) through the mouth parts and in the digestive tract of Melolonthidae larvae. This is in corroboration with the DNA analysis of F. oxysporum, which showed three different strains linked to three different species of Melolonthidae larvae, C. modesta, D. gagates and D. abderus (specific biological vectors).
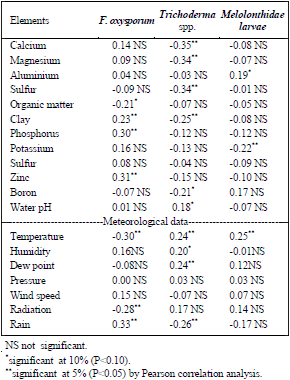
Table 2 shows significant correlations found between the presence of fungi and the evaluated parameters. F. oxysporum presented a positive relationship with percentages of clay, P and Zn, (r=0.23), (r=0.30) and (r=0.31), respectively.
Table 2 - Correlations between Melolonthidae larvae, fungi, chemical analysis of the soil and climatological data. 2012/13 – Arroio Grande, Santa Maria/RS.
Trichoderma spp. had a positive relationship with water pH (r=0.19). Melolonthidae larvae with Trichoderma spp. had a positive relationship with Al percentage (r=0.19). F. oxysporum had a positive relationship with rain (r=0.33). Trichoderma spp. had a positive relationship with temperature (r=0.24), humidity (r=0.20) and dew point (r=0.24). Melolonthidae larvae with Trichoderma spp. had a positive relationship with temperature (r=0.25).
ESCANDE et al. (1994, 2002) reported successful Trichoderma spp. inoculum dispersion by bees (Apis mellifera), leading to an 80% reduction of Sclerotinia sclerotiorum. In the present study, the induced vectoring test with Trichoderma formulation in larvae C. modesta and D. gagates led to high larval mortality rates (92.59%), together with malformations during metamorphosis from pupa to adult. Our findings indicate that there may be specific and more complex forms of interaction, competition and equilibrium, between F. oxysporum and Trichoderma spp. for colonization of the rhizosphere of host plants through specific biological vectors.
This is the first record of simultaneous dispersion of Fusarium oxysporum by Cyclocephala modesta, Dyscinetus gagates and Diloboderus abderus (Coleoptera: Melolonthidae) under soybean in Brazil.
Acknowledgments
We thanks Ivair Valmorbida in larvae Melolonthidae identification, Ricardo Harakava, for Fusarium oxysporum identification, Zaida Inês Antoniolli and Daiana Bortolluzzi Baldoni for assistance in molecular phylogenetic analysis, also Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
This article was originally published in Ciência Rural, Santa Maria, v.46, n.6, p.943-949, jun, 2016. http://dx.doi.org/10.1590/0103-8478cr20150471. This is an Open Access article distributed under the terms of the Creative Commons Attribution License. References
ACHENBACH, L.A. et al. Use of RAPD markers as a diagnostic tool for the identification of Fusarium solani isolates that cause soybean sudden death syndrome. Plant Disease, v.80, p.1228-1232, 1996. Available from: <https://www.apsnet.org/ publications/PlantDisease/BackIssues/Documents/1996Articles/ PlantDisease80n11_1228.pdf>. Acessed: Jan. 31, 2016.
ALTSCHUL, S.F. et al. Basic local alignment search tool. Journal Molecular Biology, v.215, p.403-410, 1990. Available from: <http://www.cmu.edu/bio/education/courses/03510/LectureNotes/ Altschul1990.pdf>. Acessed: Jan. 31, 2016.
BERCELLINI, N.; MALACALZA, L. Plagas y predadores en soja en el noroeste de la provincia de Buenos Aires (Arg.). Turrialba, v.44, n.4, p.244-254, 1994. Avaliable from: <http://cat.inist. fr/?aModele=afficheN&cpsidt=3603487>. Acessed: Jan. 30, 2016.
BURR, B. et al. Staheliomyces (Phallales) visited by Trigona (Apidae): melittophily in spore dispersal of an Amazonian stinkhorn? Journal of Tropical Ecology, v.12, p.441-445, 1996. Available from: <http://journals.cambridge.org/action/ displayAbstract?fromPage=online&aid=5250992&fileId =S0266467400009652>. Acessed: Jan. 31, 2016.
DOUGLAS, A.E.; BEARD, C.B. Microbial symbioses in the midgut of insects. In: LEHANE, M.J.; BILLINGSLEY, P.F. Biology of the insect midgut. London: Chapmam & Hall, 1996. p.419-429. Available from: <http://link.springer.com/chapter/10.1007/978-94- 009-1519-0_15#page-1>. Acessed: Jan. 31, 2016.
DOYLE, J.J.; DOYLE, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin, v.19, n.1, p.11-15, 1987. Available from: <http://ci.nii.ac.jp/ naid/10021087108/>. Acessed: Jan. 31, 2016.
ESCANDE, A.R. et al. Dispersión de inóculo de Trichoderma spp. Mediante abejas (Apis mellifera) para el control de la pudrición del capítulo del girasol (Sclerotinia sclerotiorum). Fitopatologia, v.29, p.35, 1994. Available from: <http://www.sidalc.net/cgibin/ wxis.exe/?IsisScript=inta2.xis&method=post&formato=2&cantid ad=1&expresion=mfn=002958>. Acessed: Jan. 31, 2016.
ESCANDE, A.R. et al. Field testing of honeybee dispersed Trichoderma spp. to manage sunflower head rot (Sclerotinia sclerotiorum). Plant Pathology, v.51, p.346-351, 2002.Available from: <http://onlinelibrary.wiley.com/doi/10.1046/ j.1365-3059.2002.00723.x/full>. Acessed: Jan. 31, 2016.
GAMA, F. de C. et al. Diversity of filamentous fungi associated with Hypothenemus hampei (Ferrari) (Coleoptera: Scolytidae) and its galleries in berries of Coffea canephora (Pierre). Neotropical Entomology, v.35, p.573-578, 2006. Available from: <http://www. scielo.br/scielo.php?pid=S1519-566X2006000500002&script=sci_ arttext>. Acessed: Jan. 31, 2016.
HARWOOD, R.F.; JAMES, M.T. Entomology in human and animal health. New York: Macmillan, 1979. 548p. Available from: <http://www.cabdirect.org/abstracts/19802256996.html>. Acessed: Jan. 31, 2016.
HIRST, J.M.; SCHEIN, R.D. Terminology of infection processes. Phytopathology, v.55, p.1157, 1965.
HUANG, Y.H.; HARTMAN, G.L. Reaction of selected soybean genotypes to isolates of Fusarium solani f. sp. glycines and their culture filtrates. Plant Disease, v.82, p.999-1002, 1998. Available from: <http://apsjournals.apsnet.org/doi/pdfplus/10.1094/ PDIS.1998.82.9.999>. Acessed: Jan. 31, 2016.
ISMAIL, M.A.; ABDEL-SATER, M.A. Fungi associated with the Egyptian cotton leafworm Spodoptera littoralis Boisduval. Mycopathology, v.124, p.79-86, 1993. Avaliable from: <http://link.springer.com/article/10.1007%2FBF01103106>. Acessed: Jan. 31, 2016.
KUMAR, N.S. et al. Histology and fungal flora of shote-hole borer beetles (Xyleborus fornicatus) galleries in tea (Camellia sinensis). Journal of the National Science Council of Sri Lanka, v.26, p.195-207, 1998. Available from: <http://jnsfsl.sljol.info/article/ abstract/10.4038/jnsfsr.v26i3.5002/>. Acessed: Jan. 31, 2016.
LE PELLEY, R.H. Pests of coffee. London: Longman, 1968. 590p. Avaliable from: <http://www.cabdirect.org/abstracts/19690500391.html;jsessionid=7102B5D659C01B9C5C BAE6616E0DB994>. Acessed: Jan. 31, 2016.
LESLIE, J.F.; SUMMERELL, B.A. The Fusarium laboratory manual. Oxford, UK: Blackwell Publishing, 2006. 388p. Avaliable from: <http://www.wiley.com/WileyCDA/WileyTitle/ productCd-0813819199.html>. Acessed: Jan. 31, 2016.
MORALES-RAMOS, J.A. et al. Symbiotic relationship between Hypothenemus hampei (Coleoptera: Scolytidae) and Fusarium solani (Moniliales: Tuberculariaceae). Annals of the Entomological Society of America, v.93, p.541-547, 2000. Avaliable from: <http://aesa.oxfordjournals.org/content/93/3/541. abstract>. Acessed: Jan. 31, 2016.
NELSON, P.E. et al. Fusarium species: an illustrated manual for identification. Philadelfia: Pennsylvania State University, 1983. 193p.
OLIVEIRA, M.L.; MORATO, E.F. Stingless bees (Hymenoptera, Meliponini) feeding on stinkhorn spores (Fungi, Phallales): robbery or dispersal? Revista Brasileira de Zoologia, v.17, n.3, p.881-884, 2000. Available from: <http://www.scielo.br/pdf/ rbzool/v17n3/v17n3a25.pdf>. Acessed: Jan. 31, 2016.
PARDO-LOCARNO, L.C. et al. Structure and composition of the White grub complex (Coleoptera: Scarabaeidae) in agroecological systems of northern cauca, Colômbia. Florida Entomologist, v.88, n.4, p.355-363, 2005. Available from: <http://www.bioone. org/doi/abs/10.1653/00154040(2005)88%5B355:SACOTW%5D2 .0.CO%3B2>. Acessed: Jan. 31, 2016.
R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2014. Available from: <http://www.R-projetct.org>. Acessed: May 20, 2015.
SALGADO-NETO, G. et al. First report of the occurrence of Ophiocordyceps melolonthae (Ascomycota: Hypocreales: Ophiocordycipitaceae) in larvae of Diloboderus abderus Sturm (Coleoptera: Melolonthidae) in Brazil. Biota Neotropica, v.15, n.2, p.1-4, sept. 2015. Available from: <http://www.scielo.br/ scielo.php?pid=S1676-06032015000200402&script=sci_arttext>. Acessed: nov30, 2015. doi: 10.1590/1676-06032015010814.
SIEGEL, S.; CASTELLAN, Jr, N.J. Estatística não paramétrica para as ciências do comportamento. São Paulo: Artmed Bookman, 2006, Reimpressão 2008. 448 p.
SILVA-WERNECK, J.O. et al. Técnica de criação de Diabrotica speciosa (Germ.) (Coleoptera: Chrysomelidae) para bioensaios com bacilos e fungos entomopatogênicos. Anais da Sociedade Entomológica do Brasil, v.24, n.1, p.45-52, 1995.
SUTTON, J.C.; PENG, G. Manipulation and vectoring of biocontrol organisms to manage foliage and fruit diseases in cropping systems. Annual Review of Phytopathology, v.31, p.473-493, 1993. Available from: <http://garfield.library.upenn. edu/histcomp/annualreviews/ann-revphytopatho/node/642.html>. Acessed: Jan. 31, 2016.
SCHMITZ, A.; RIESNER, D. Purification of nucleic acids by selective precipitation with polyethylene glycol 6000. Analytical Biochemistry, v.354, p.311-313, 2006. Available from: <http:// www.sciencedirect.com/science/article/pii/S0003269706001795>. Acessed: Jan. 31, 2016.
TAMURA, K.; NEI, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, v.10, p.512-526, 1993.
TAMURA, K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, v.28, n.10, p.2731-2739, 2011. Available from: <http:// www.ncbi.nlm.nih.gov/pmc/articles/PMC3203626/>. Acessed: Nov. 30, 2015. doi: 10.1093/molbev/msr121.
TUNO, N. Spore dispersal of Dictyophora fungi (Phallaceae) by flies. Ecological Research, v.13, p.7-15, 1998. Available from: <https:// www.researchgate.net/profile/Nobuko_Tuno/publication/36435639_ Spore_dispersal_of_Dictyophora_fungi_(Phallaceae)_by_Flies/ links/00b7d51bef32ae90ef000000.pdf>. Acessed: Jan. 31, 2016.
VENTURA, J.A. Taxonomia de Fusarium e seus segregados. Parte II - Chaves para identificação. In: Fernandes, J.M.; Prestes, A.M.; Picinini, C.E. Revisão anual de patologia de plantas. Editora LUZ, W.C., 2000. v.8, p.303-338.
WHITE, T.J. et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: INNIS, M.A. et al. PCR Protocols: a guide to methods and applications. New York: Academic, 1990. p.315-322.
WHITNEY, H.S. Relationships between bark beetles and symbiotic organisms. In: MILTON, J.B.; STUREEON, R.B. Bark beetles in North American conifers: a system for study of evolutionary biology. Austin: University of Texas, 1982. p.183-211.
ZAMBOLIM, L. et al. Controle de doenças de plantas hortaliças. Viçosa: UFV, 2000. 444p. Avaliable from: <http:// www.sidalc.net/cgi-bin/wxis.exe/?IsisScript=AGB.xis&meth od=post&formato=2&cantidad=1&expresion=mfn=203397>. Acessed: Jan. 31, 2016.
ZOBERI, M.; GRACE, K. Fungi associated with subterranean termite Reticulitermes flavipes (Kollar) in Ontario. Mycology, v.82, p.289-294, 1990. Available from: <http://www.jstor.org/sta ble/3759899?origin=crossref&seq=1#page_scan_tab_contents>. Acessed: Jan. 31, 2016.







.jpg&w=3840&q=75)