Introduction
Mycotoxins are secondary metabolites of low molecular weights produced by certain strains of filamentous fungi such as Aspergillus, Fusarium and Penicillium, which invade crops in the field and may grow on foods during storage under favorable conditions of temperature and humidity. The most common mycotoxins are aflatoxins, fumonisins, ochratoxin A, trichothecenes, zearalenone, and out of which aflatoxins (AF) commonly contaminate a wide variety of tropical and subtropical food/feedstuffs (Katole et al., 2013). No region of world escapes with the problem of mycotoxins and according to Lawler and Lynch (2005) the mycotoxins are estimated to affect as much as 20% of world’s crop each year. Crops grown under warm and moist weather in tropical or subtropical countries are especially more prone to aflatoxin contamination than those in temperate zones. Grains stored under high moisture or humidity at warm temperatures and inadequately dried can potentially become contaminated. Initial growth of fungi in grains can form sufficient moisture from metabolism to allow for further growth and mycotoxin formation. Aflatoxin deteriorates the quality of the feed; therefore it has tremendous economic impact on the poultry industry (Katole et al., 2013).
It is shown by several authors that the trace elements as inorganic form are important factors that influence the fungal growth and AF production in feed ingredient (Jones et al., 1984). Several studies report that the organic or chelated minerals are more bioavailable to animals and poultry (Kratzer et al., 1969; Wedekind et al., 1992) than its inorganic counterpart and hence one can expect more counteracting effect of this form of minerals against AF than inorganic form. Even though several reports are available on the effect of inorganic trace metals on fungal growth and AF production in feed ingredients, literature regarding the effect of supplemental chelated minerals on fungal growth and AF production are scanty. The objective of present investigation was to study the effect of certain chelated and inorganic trace minerals on fungal growth and aflatoxin production in liquid media
Materials and Methods
Preparation of basal and experimental substrate:Fifty gm of good quality maize, free from possible adulterants was taken in each 250 ml conical flask, plugged with cotton and autoclaved at 15 1b psi for 15 minutes and cooled. Nine ml of distilled water was taken in test tubes and autoclaved at 15 1b psi for 15 minutes and cooled. Then, one ml of trace mineral solution was added to the sterilized distilled water under sterile condition and the test tubes were again sterilized by cold sterilization by keeping the test tubes in maximum slanting position directly under UV light of laminar flow for 3 hours. Then, the 10 ml of sterilized distilled water with trace minerals were added to the autoclaved maize under sterile condition and the flasks were shaken well for the uniform distribution of minerals. For control flasks, 10 ml of sterile distilled water was added. All the flasks were individually marked according to the treatment.
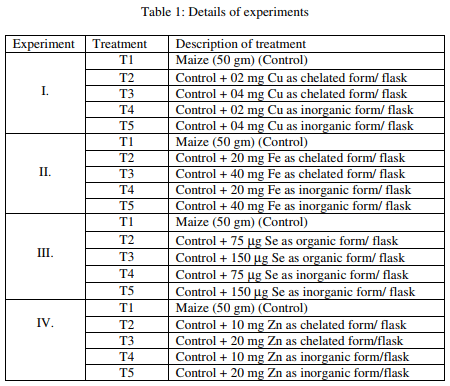
Experimental design: In experiment I, II, III and IV, the copper (Cu), iron (Fe), selenium (Se) and zinc (Zn) were supplemented, respectively. The details of supplementation in these experiments are given below. Each treatment was replicated by six observations and experimental design followed was Completely Randomized Design (CRD) (Table 1).
Inoculation of mould and incubation:The experimental substrates in flasks were inoculated with one ml of fungal spore suspension containing 106 -107 spores/ ml of PBS under sterile condition. The flasks were incubated at 28°C +1 °C for 7 days in BOD incubator. Regular shaking was done to avoid sticking of the maize and to facilitate distribution of minerals. After incubation period, the flasks containing cultured maize were again autoclaved. The cultured maize was dried in hot air oven at 800C for 24 hours, grinded to powder form and stored in individual polythene bags with proper labeling for analysis of AF concentration.
Analysis of aflatoxin production:The extraction of AF was done as per the procedure described by Pons et al. (1966) in which aqueous acetone was used for the extraction of the toxin, followed by TLC analysis of AF.
Results and Discussion
The results revealed that AFB1 production in maize between treatments exerted a highly significant (P<0.01) effect due to excess dietary concentrations of the minerals (Table 2). Experiment 1 indicated that the maize amended with 2 or 4 mg inorganic Cu (per 50 gm maize) (T4 and T5) had significantly higher AFB1 compared to that of control (T1). The AFB1 content of group T3 was significantly lower than that of control, however, the AFB1 content of group T2 was statistically similar to that of control. In Experiment II, the AFB1 production was significantly higher in maize amended without or with 20 mg of chelated or inorganic Fe (per 50 gm maize) ie. T1, T2, T4 than maize amended with 40 mg of chelated or inorganic Fe (T3, T5).
The results of Experiment III revealed that the AFB1 in maize amended with lower organic Se (1.5 ppm) or higher inorganic Se (3 ppm) ie. T2 and T5, respectively was significantly higher followed by lower inorganic Se (1.5 ppm) or higher organic Se (3 ppm) than the maize without Se supplementation. The result of Experiment IV revealed that the AFB1 in maize amended with 10 and 20 mg inorganic Zn (per 50 gm maize) ie. T4 and T5 was significantly higher than AFB1 of maize unamended with Zn (T1) whereas, the AFB1 in maize amended with 10 and 20 mg chelated Zn (T2, T3) was significantly lower than control. However, there was no significant difference between two inorganic Zn levels or chelated Zn levels.
The result revealed that maize amended with Cu or Zn in inorganic form had higher AFB1 production, but the maize amended with Cu, Zn in chelate form had either decreased or equal AFB1 production, as compared to control. This result implies that Cu or Zn in inorganic form was utilized by the fungi and subsequently increased AFB1 production and Cu or Zn in chelated form is unavailable to fungi for AF production. In congruency with the result, increased AF production due to excess Zn in feed or feed ingredients are recorded by many authors (Aziz et al., 2000; Ginting and Barlean, 1987; Failla et al., 1986; Jones et al., 1984; Lilliehoj et al., 1974). Similarly, increased AF production due to excess Cu in feed ingredients was noticed by Aziz et al. (2000) and Failla et al. (1986). In contrary to this, Chulze et al. (1987) could not find evidence on the influence of Zn on AF production in sunflower seeds. However, Hensarling et al. (1983) found that addition of ZnSo4 to soybean meal inhibited AF production and supplementation with sodium phytate relieved this inhibition. Similarly, Lillehoj et al. (1974) found a reduction in AF elaboration due to addition of 500 µg of Cu/ gm of corn germ. However, the studies on effect of minerals in chelated form on AF production are rare. It has been reported that although the Zn content of grains is sufficient to permit AF formation, the metal is generally complexes with phytic acid, thus preventing the release of AF (Gupta and Venkitasubramanian, 1975). The decrease in AF level in maize amended with Zn and Cu chelate than control might possibly due to mild propionic acid added as a part of the chelated Zn and Cu product.
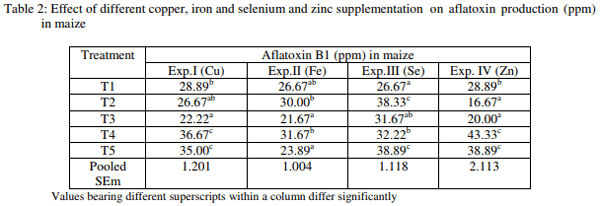
The AF level in maize amended with Fe in both organic and inorganic form did not differ significantly with AF level in maize unamended with Fe. This result implies that Fe supplementation in any form is not utilized by the fungi for AF production, while the higher level of AF is detrimental for AF production. In contrary to the result, Aziz et al. (2000) found that 100 ppm of Fe enhance fungal growth and AF production in corn. However the studies on effect Fe chelate on AF production are very rare.
The supplementation of maize with either organic or inorganic Se significantly increased the AF levels and there was no difference in the utilization of organic or inorganic Se by the fungi. The result suggests that the supplementation of Se in feed to counteract aflatoxicosis in vivo should be considered carefully for the effect of Se on its utilization by contaminating fungi and subsequent AF production in feed.
Conclusion
It was concluded that the fungi may not utilize the minerals in chelated form as efficiently as in inorganic form, for aflatoxin synthesis in feed. The excess selenium either in organic or inorganic form was found to increase the aflatoxin production in maize.
This article was originally published in Journal of Poultry Science and Technology, October-December 2013, Vol 1, Issue 1, Pages 13-16.