Bioactive Metabolites from the Endophytic Fungus Aspergillus sp. SPH2
In the current study, an ethyl acetate extract from the endophytic fungus Aspergillus sp. SPH2 isolated from the stem parts of the endemic plant Bethencourtia palmensis was screened for its biocontrol properties against plant pathogens (Fusarium moniliforme, Alternaria alternata, and Botrytis cinerea), insect pests (Spodoptera littoralis, Myzus persicae, Rhopalosiphum padi), plant parasites (Meloidogyne javanica), and ticks (Hyalomma lusitanicum). SPH2 gave extracts with strong fungicidal and ixodicidal effects at different fermentation times. The bioguided isolation of these extracts gave compounds 1–3. Mellein (1) showed strong ixodicidal effects and was also fungicidal. This is the first report on the ixodicidal effects of 1. Neoaspergillic acid (2) showed potent antifungal effects. Compound 2 appeared during the exponential phase of the fungal growth while neohydroxyaspergillic acid (3) appeared during the stationary phase, suggesting that 2 is the biosynthetic precursor of 3. The mycotoxin ochratoxin A was not detected under the fermentation conditions used in this work. Therefore, SPH2 could be a potential biotechnological tool for the production of ixodicidal extracts rich in mellein.
Keywords: endophyte; Aspergillus ochraceous; antifungal; neoaspergillic acid; ixodicidal; mellein.

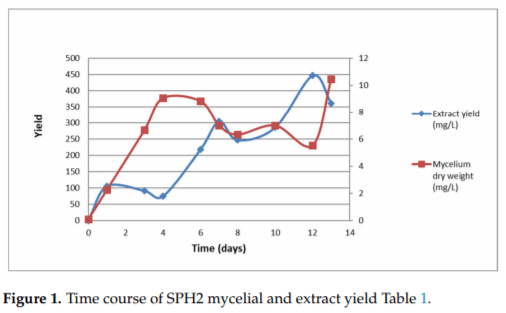
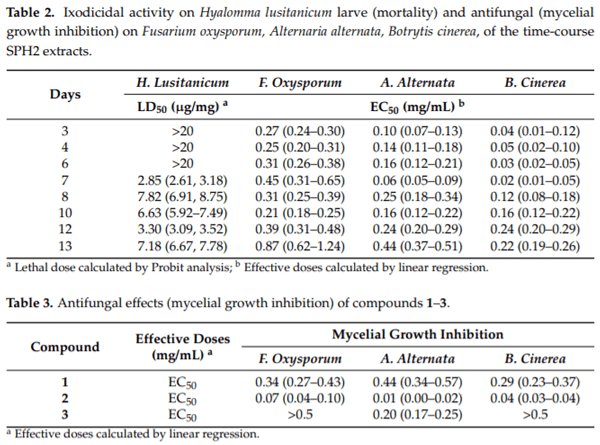


- Tawfike, A.F.; Tate, R.; Abbott, G.; Young, L.; Viegelmann, C.; Schumacher, M.; Han, B.W.; Edrada-Ebel, R. Metabolomic Tools to Assess the Chemistry and Bioactivity of EndophyticAspergillusStrain. Chem. Biodivers. 2017, 14, e1700040. [CrossRef] [PubMed]
- Strobel, G.; Daisy, B. Bioprospecting for Microbial Endophytes and Their Natural Products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [CrossRef] [PubMed]
- Firáková, S.; Šturdíková, M.; Múckov?á, M. Bioactive secondary metabolites produced by microorganisms associated with plants. Biologia 2007, 62, 251–257. [CrossRef]
- Germaine, K.; Keogh, E.; Garcia-Cabellos, G.; Borremans, B.; Van Der Lelie, D.; Barac, T.; Oeyen, L.; Vangronsveld, J.; Moore, F.P.; Moore, E.R.; et al. Colonisation of poplar trees by gfp expressing bacterial endophytes. FEMS Microbiol. Ecol. 2004, 48, 109–118. [CrossRef]
- Wang, Y.; Dai, C.-C. Endophytes: A potential resource for biosynthesis, biotransformation, and biodegradation. Ann. Microbiol. 2011, 61, 207–215. [CrossRef]
- Guo, B.; Wang, Y.; Sun, X.; Tang, K. Bioactive natural products from endophytes: A review. Appl. Biochem. Microbiol. 2008, 44, 136–142. [CrossRef]
- Andrés, M.F.; Diaz, C.E.; Giménez, C.; Cabrera, R.; González-Coloma, A. Endophytic fungi as novel sources of biopesticides: The Macaronesian Laurel forest, a case study. Phytochem. Rev. 2017, 16, 1009–1022. [CrossRef]
- Morales-Sánchez, V.; Fe Andrés, M.; Díaz, C.E.; González-Coloma, A. Factors Affecting the Metabolite Productions in Endophytes: Biotechnological Approaches for Production of Metabolites. Curr. Med. Chem. 2020, 27, 1855–1873. [CrossRef]
- Higginbotham, S.; Arnold, A.E.; Ibañez, A.; Spadafora, C.; Coley, P.D.; Kursar, T.A. Bioactivity of Fungal Endophytes as a Function of Endophyte Taxonomy and the Taxonomy and Distribution of Their Host Plants. PLoS ONE 2013, 8, e73192. [CrossRef]
- Harrison, J.G.; Griffin, E.A. The diversity and distribution of endophytes across biomes, plant phylogeny and host tissues: How far have we come and where do we go from here? Environ. Microbiol. 2020, 22, 2107–2123. [CrossRef]
- The Macaronesian Region. Available online: https://ec.europa.eu/environment/nature/natura2000/biogeog_regions/macaronesian/index_en.htm (accessed on 12 December 2020).
- Fraga, B.M.; Díaz, C.E.; Amador, L.J.; Reina, M.; Santana, O.; Gonzalez-Coloma, A. Bioactive compounds from transformed root cultures and aerial parts of Bethencourtia hermosae. Phytochemistry 2014, 108, 220–228. [CrossRef] [PubMed]
- Portero, A.G.; González-Coloma, A.; Reina, M.; Díaz, C.E. Plant-defensive sesquiterpenoids from Senecio species with biopesticide potential. Phytochem. Rev. 2012, 11, 391–403. [CrossRef]
- Nordenstam, B. “Canariothamnus B.” Nord., a new genus of the Compositae-Senecioneae, endemic to the Canary Islands. Comp. Newsl. 2006, 44, 24–31.
- Kumar, S.; Kaushik, N. Endophytic Fungi Isolated from Oil-Seed Crop Jatropha curcas Produces Oil and Exhibit Antifungal Activity. PLoS ONE 2013, 8, e56202. [CrossRef] [PubMed]
- Kumar, S.; Kaushik, N.; Edrada-Ebel, R.; Ebel, R.; Proksch, P. Isolation, characterization, and bioactivity of endophytic fungi of Tylophora indica. World J. Microbiol. Biotechnol. 2011, 27, 571–577. [CrossRef]
- Parra, A.J.E.; Cuca, L.E.; González-Coloma, A. Antifungal and phytotoxic activity of benzoic acid derivatives from inflorescences of Piper cumanense. Nat. Prod. Res. 2019, 1–9. [CrossRef] [PubMed]
- Ruiz-Vásquez, L.; Olmeda, A.S.; Zúñiga, G.; Villarroel, L.; Echeverri, L.F.; González-Coloma, A.; Reina, M. Insect Antifeedant and Ixodicidal Compounds from Senecio adenotrichius. Chem. Biodivers. 2017, 14, e1600155. [CrossRef] [PubMed]
- Wang, J.; Wang, G.; Zhang, Y.; Zheng, B.; Zhang, C.; Wang, L. Isolation and identification of an endophytic fungus Pezicula sp. in Forsythia viridissima and its secondary metabolites. World J. Microbiol. Biotechnol. 2014, 30, 2639–2644. [CrossRef] [PubMed]
- Cimmino, A.; Cinelli, T.; Masi, M.; Reveglia, P.; Da Silva, M.A.; Mugnai, L.; Michereff, S.J.; Surico, G.; Evidente, A. Phytotoxic Lipophilic Metabolites Produced by Grapevine Strains of Lasiodiplodia Species in Brazil. J. Agric. Food Chem. 2017, 65, 1102–1107. [CrossRef] [PubMed]
- Yamazaki, M.; Maebayashi, Y.; Miyaki, K. Isolation of a new type of pyrazine metabolite from Aspergillus sp. Chem. Pharm. Bull. 1972, 20, 2274–2276. [CrossRef]
- Maebayashi, Y.; Sumita, M.; Fukushima, K.; Yamazaki, M. Isolation and structure of red pigment from Aspergillus sp. Wilh. Chem. Pharm. Bull. 1978, 26, 1320–1322. [CrossRef]
- Assante, G.; Camarda, L.; Locci, R.; Merlini, L.; Nasini, G.; Papadopoulos, E. Isolation and structure of red pigments from Aspergillus flavus and related species, grown on a differential medium. J. Agric. Food Chem. 1981, 29, 785–787. [CrossRef]
- Bao, J.; Wang, J.; Zhang, X.Y.; Nong, X.H.; Qi, S.H. New furanone derivatives and alkaloids from the co-culture of marine derived fungi Aspergillus sclerotiorum and Penicillium citrinum. Chem. Biodivers. 2017, 14, e1600327. [CrossRef] [PubMed]
- Luo, P.; Shao, G.; Zhang, S.; Zhu, L.; Ding, Z.; Cai, L. Secondary metabolites of endophytic fungus Aspergillus sp. SX-C7 from Selaginella stauntoniana. Zhong Cao Yao 2020, 51, 3856–3862.
- Kamel, N.M.; Abdel-Motaal, F.F.; El-Zayat, S.A. Endophytic fungi from the medicinal herb Euphorbia geniculata as a potential source for bioactive metabolites. Arch. Microbiol. 2020, 202, 247–255. [CrossRef]
- Cheng, Z.; Ke, Z.; Wu, Y. Study on secondary metabolites of endophytic fungus Aspergillus sp. from Polygonatum cyrtonema. Zhong Cao Yao 2019, 50, 5424–5428.
- Attia, E.Z.; Farouk, H.M.; Abdelmohsen, U.R.; El-Katatny, M.H. Antimicrobial and extracellular oxidative enzyme activities of endophytic fungi isolated from alfalfa (Medicago sativa) assisted by metabolic profiling. S. Afr. J. Bot. 2020, 134, 156–162. [CrossRef]
- Paynor, K.A.; David, E.S.; Valentino, M.J.G. Endophytic fungi associated with bamboo as possible sources of single cell protein using corn cob as a substrate. Mycosphere 2016, 7, 139–147. [CrossRef]
- Da Silva, D.M.; de Sousa Carvalho, F.R.; Moura, A.G.; Martins, L.; Ferreira, P.M.; Peron, A.P. Cytotoxic action of the stem aqueous extract of the stem of Cereus jamacaru DC. (mandacaru). Rev. Cuba. Plantas Med. 2015, 20, 226–234.
- Bezerra, J.D.; Nascimento, C.C.; Barbosa, R.D.N.; Da Silva, D.C.; Svedese, V.M.; Silva-Nogueira, E.B.; Gomes, B.S.; Paiva, L.M.; Motta, C.M.D.S. Endophytic fungi from medicinal plant Bauhinia forficata: Diversity and biotechnological potential. Braz. J. Microbiol. 2015, 46, 49–57. [CrossRef]
- Sudheep, N.M.; Sridhar, K.R. Non-mycorrhizal fungal endophytes in two orchids of Kaiga forest (Western Ghats), India. J. For. Res. 2012, 23, 453–460. [CrossRef]
- Mahmoud, A.-L.E. Mycotoxin-producing potential of fungi associated with qat (Catha edulis) leaves in yemen. Folia Microbiol. 2000, 45, 452–456. [CrossRef]
- Guo, S.; Mao, W.; Yan, M.; Zhao, C.; Li, N.; Jimiao, S.; Lin, C.; Liu, X.; Guo, T.; Guo, T.; et al. Galactomannan with novel structure produced by the coral endophytic fungus Aspergillus sp. Carbohydr. Polym. 2014, 105, 325–333. [CrossRef] [PubMed]
- Cui, C.M.; Li, X.M.; Meng, L.; Li, C.S.; Huang, C.G.; Wang, B.G. 7-Nor-ergosterolide, a pentalactone-containing norsteroid and related steroids from the marine-derived endophytic Aspergillus sp. EN-31. J. Nat. Prod. 2010, 73, 1780–1784. [CrossRef] [PubMed]
- Cui, C.M.; Li, X.M.; Li, C.S.; Sun, H.F.; Gao, S.S.; Wang, B.G. Benzodiazepine Alkaloids from Marine-Derived Endophytic Fungus Aspergillus sp. Helv. Chim. Acta 2009, 92, 1366–1370. [CrossRef]
- Reveglia, P.; Masi, M.; Evidente, A. Melleins—Intriguing Natural Compounds. Biomolecules 2020, 10, 772. [CrossRef] [PubMed]
- Visagie, C.; Varga, J.; Houbraken, J.; Meijer, M.; Kocsubé, S.; Yilmaz, N.; Fotedar, R.; Seifert, K.; Frisvad, J.; Samson, R. Ochratoxin production and taxonomy of the yellow aspergilli (Aspergillus section Circumdati). Stud. Mycol. 2014, 78, 1–61. [CrossRef] [PubMed]
- Zhao, J.H.; Zhang, C.; Wang, L.W.; Wang, J.Y. Bioactive secondary metabolites from Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam. World J. Microbiol. Biotechnol. 2012, 28, 2107–2112. [CrossRef]
- Cimmino, A.; Maddau, L.; Masi, M.; Linaldeddu, B.T.; Evidente, A. Secondary metabolites produced by Sardiniella urbana, a new emerging pathogen on European hackberry. Nat. Prod. Res. 2019, 33, 1862–1869. [CrossRef]
- Wang, Q.; Yang, X.-Q.; Miao, C.-P.; Xu, L.-H.; Ding, Z.; Yang, Y.-B.; Zhao, L.-X. A New Pair of Pentaketide Diastereoisomers from Aspergillus melleus YIM PHI001. Rec. Nat. Prod. 2018, 12, 216–221. [CrossRef]
- Hori, M.; Aoki, Y.; Shinoda, K.; Chiba, M.; Sasaki, R. Wood volatiles as attractants of the confused flour beetle, Tribolium confusum (Coleoptera: Tenebrionidae). Sci. Rep. 2019, 9, 1–8. [CrossRef] [PubMed]
- Voegtle, H.L.; Jones, T.H.; Davidson, D.W.; Snelling, R.R. E-2-Ethylhexenal, E-2-Ethyl-2-Hexenol, Mellein, and 4-Hydroxymellein in Camponotus Species from Brunei. J. Chem. Ecol. 2008, 34, 215–219. [CrossRef] [PubMed]
- Blum, M.S.; Morel, L.; Fales, H.M. Chemistry of the mandibular gland secretion of the ant Camponotus vagus. Comp. Biochem. Physiol. Part B Comp. Biochem. 1987, 86, 251–252. [CrossRef]
- Mitaka, Y.; Mori, N.; Matsuura, K. A termite fungistatic compound, mellein, inhibits entomopathogenic fungi but not egg-mimicking termite ball fungi. Appl. Èntomol. Zool. 2019, 54, 39–46. [CrossRef]
- Kendagor, A.C.; Langat, M.K.; Cheplogoi, P.K.; Omolo, J.O. Larvicidal activity of mellein from cultures of an ascomycete Pezicula livida against Aedes aegypti. Int. J. Life Sci. Biotechnol. Pharma. Res. 2013, 2, 70–80.
- Sajid, M.; Kausar, A.; Iqbal, A.; Abbas, H.; Iqbal, Z.; Jones, M. An insight into the ecobiology, vector significance and control of Hyalomma ticks (Acari: Ixodidae): A review. Acta Trop. 2018, 187, 229–239. [CrossRef]
- Chitimia-Dobler, L.; Schaper, S.; Rieß, R.; Bitterwolf, K.; Frangoulidis, D.; Bestehorn, M.; Springer, A.; Oehme, R.M.; Drehmann, M.; Lindau, A.; et al. Imported Hyalomma ticks in Germany in 2018. Parasites Vectors 2019, 12, 1–9. [CrossRef]
- Hansford, K.M.; Carter, D.; Gillingham, E.L.; Hernandez-Triana, L.M.; Chamberlain, J.; Cull, B.; McGinley, L.; Phipps, L.P.; Medlock, J.M. Hyalomma rufipes on an untraveled horse: Is this the first evidence of Hyalomma nymphs successfully moulting in the United Kingdom? Ticks Tick-Borne Dis. 2019, 10, 704–708. [CrossRef]
- Buczek, A.M.; Buczek, W.; Bartosik, K. The Potential Role of Migratory Birds in the Rapid Spread of Ticks and Tick-Borne Pathogens in the Changing Climatic and Environmental Conditions in Europe. Int. J. Environ. Res. Public Health 2020, 17, 2117. [CrossRef]
- Grandi, G.; Chitimia-Dobler, L.; Choklikitumnuey, P.; Strube, C.; Springer, A.; Albihn, A.; Jaenson, T.; Omazic, A. First records of adult Hyalomma marginatum and H. rufipes ticks (Acari: Ixodidae) in Sweden. Ticks Tick-Borne Dis. 2020, 11, 101403. [CrossRef]
- Lebar, M.D.; Mack, B.M.; Carter-Wientjes, C.H.; Gilbert, M.K. The aspergillic acid biosynthetic gene cluster predicts neoaspergillic acid production in Aspergillus section Circumdati. World Mycotoxin J. 2019, 12, 213–222. [CrossRef]
- Xu, X.-Y.; He, F.; Zhang, X.; Bao, J.; Qi, S. New mycotoxins from marine-derived fungus Aspergillus sp. SCSGAF0093. Food Chem. Toxicol. 2013, 53, 46–51. [CrossRef] [PubMed]
- Chen, X.W.; Li, C.W.; Hua, W.; Wu, C.J.; Cui, C.B.; Zhu, T.J.; Gu, Q.Q. Metabolites of Aspergillus sp. 16-02-1 isolated from a deep sea sediment and preliminary test of their antitumor and antifungal activities. Chin. J. Mar. Drugs 2013, 32, 1–10.
- Cardoso-Martínez, F.; de la Rosa, J.M.; Díaz-Marrero, A.R.; Darias, J.; D’Croz, L.; Cerella, C.; Diederich, M.; Cueto, M. Oxi-moaspergillimide, a fungal derivative from a marine isolate of Aspergillus sp. Eur. J. Org. Chem. 2015, 10, 2256–2261. [CrossRef]
- Zhu, F.; Chen, G.; Chen, X.; Huang, M.; Wan, X. Aspergicin, a new antibacterial alkaloid produced by mixed fermentation of two marine-derived mangrove epiphytic fungi. Chem. Nat. Compd. 2011, 47, 767–769. [CrossRef]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and Human Health Risk: A Review of the Evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [CrossRef]








