Beneficial Properties of Probiotic Yeast Saccharomyces boulardii
Saccharomyces boulardii is a unique probiotic and biotherapeutic yeast, known to survive in gastric acidity and it is not adversely affected or inhibited by antibiotics or does not alter or adversely affect the normal microbiota. S. boulardii has been utilized worldwide as a probiotic supplement to support gastrointestinal health. The multiple mechanisms of action of S. boulardii and its properties may explain its efficacy and beneficial effects in acute and chronic gastrointestinal diseases that have been confirmed by clinical trials. Caution should be taken in patients with risk factors for adverse events. Its potential application in various dairy foods could offer an alternative probiotic product to people suffering from antibiotic-associated diarrhea. This review discusses the evidence for efficacy and safety of S. boulardii as a probiotic for the prevention and therapy of gastrointestinal disorders in humans.
Key words: Saccharomyces boulardii, efficacy, safety, probiotic.
1. Andrade Arau´jo, E., dos Santos Pires, A. C., Soares Pinto, M., Jan, G., de Carvalho, A. F. (2012). Probiotics in dairy fermented products. In Probiotics. Ed. E. Rigobelo, InTech, DOI: 10.5772/51939 (http://www.intechopen.com/books/probiotics/pr obiotics-in-dairy-fermented-products).
2. Beniwal, R.S., Arena, V.C., Thomas, L., Narla, S., Imperiale, T.F., Chaudhry, R.A., Ahmad, U. A. (2003). A randomized trial of yogurt for prevention of antibiotic-associated diarrhea. Digestive Diseases and Sciences, 48, 2077– 2082.
3. Billoo, A.G., Memon, M. A.,Khaskheli, S.A., Murtaza, G., Iqbal, K., Saeed Shekhani, M., Siddiqi, A.Q. (2006). Role of a probiotic (Saccharomyces boulardii ) in management and prevention of diarrhea. World Journal of Gastroenterology, 12, 4557-4560.
4. Buts, J.P., de Keyser, N. D. (2006). Effects of Saccharomyces boulardii on intestinal mucosa. Digestive Diseases and Sciences, 51, 1485– 1492.
5. Cassone, M., Serra, P., Mondello, P., Girolamo, A., Scafetti, S., Pistella, E., Venditti, M. (2003). Outbreak of Saccharomyces cerevisiae subtype boulardii fungemia in patients neighboring those treated with a probiotic preparation of the organism. Journal of Clinical Microbiology, 41, 5340–5343.
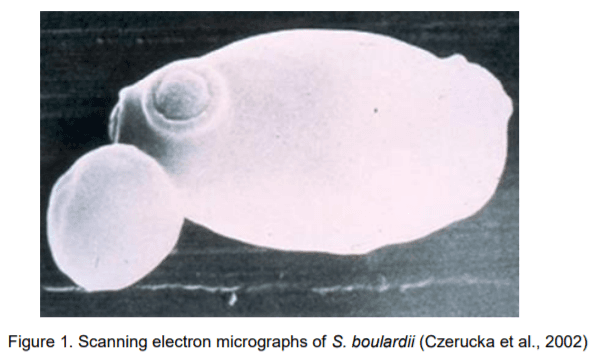
6. Czerucka, D., Piche, T., Rampal, P. (2007). Review article: yeast as probiotics – Saccharomyces boulardii. Alimentary Pharmacology & Therapeutics, 26, 767–778.
7. Czerucka, D., Rampal, P. (2002). Experimental effects of Saccharomyces boulardii on diarrheal pathogens. Microbes and Infection, 4, 733–739.
8. FAO/WHO (2002). Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. London, ON, Canada, Food and Agriculture Organization/World Health Organization.
9. Graff, S., Chaumeil, J.C., Boy, P., Lai-Kuen, R., Charrueau, C. (2008). Formulations for protecting the probiotic Saccharomyces boulardii from degradation in acidic condition. Biological and Pharmaceutical Bulletin, 31 (2), 266—272.
10. Guslandi, M., Mezzi, G., Sorghi, M., Testoni, P. A. (2000). Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Digestive Diseases and Sciences, 45, 1462–1464.
11. Guslandi, M., Giollo, P., Testoni, P. A. (2003). A pilot trial of Saccharomyces boulardii in ulcerative colitis. European Journal of Gastroenterology and Hepatology, 15 (6), 697-698.
12. Hamilton-Miller, J.M.T. (2004). Probiotics and prebiotics in the elderly. Postgraduate Medical Journal, 80, 447-451.
13. Hickson, M. (2011). Probiotics in the prevention of antibiotic-associated diarrhoea and Clostridium difficile infection. Therapeutic Advances in Gastroenterology, 4 (3), 185–197.
14. Hill, C., Guarner, F., Reid., R, Gipson, G.R., Merenstein, D.J., Pot, B., Morelli, L., Canani, R. B., Flint, H.J., Salminen, S., Calder, P.C., Sanders, M.E. (2014). Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology, 11, 506–514.
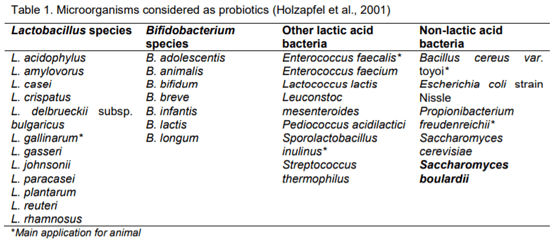
15. Holzapfel, W.H., Haberer, P., Geisen, R., Björkroth, J., Schillinger, U. (2001). Taxonomy and important features of probiotic microorganisms in food and nutrition. The American Journal of Clinical Nutrition, 73, 365S–73S.
16. Karaolis, C., Botsaris, G., Pantelides, I., Tsaltas, D. (2013). Potential application of Saccharomyces boulardii as a probiotic in goat’s yoghurt: survival and organoleptic effects. International Journal of Food Science and Technology, 48, 1445–1452.
17. Kelesidis, T., Pothoulakis, C. (2012). Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therapeutic Advances in Gastroenterology, 5,111-25.
18. ICZN (2014). What is a nomen nudum. International Commission on Zoological Nomenclature, London, UK (http://iczn.org/content/what-nomen-nudum).
19. Krasowska, A., Murzyn, A., Dyjankiewicz, A., Lukaszewicz, M., Dziadkowiec, D. (2009). The antagonistic effect of Saccharomyces boulardii on Candida albicans filamentation, adhesion and biofilm formation. FEMS Yeast Research, 9, 1312-1321.
20. Lourens-Hattingh, A., Viljoen, B.C. (2001). Growth and survival of a probiotic yeast in dairy products. Food Research International, 34, 791-796.
21. Marcia, L.B. (2009). Saccharomyces boulardii as a probiotic for children. Pediatric Pharmacolotherapy, 15 (7), 1- 5.
22. McFarland, L.V., Bernasconi, P. (1993). Saccharomyces boulardii: A review of an innovative biotherapeutic agent. Microbial Ecology in Health and Disease, 6, 157-171.
23. McFarland, L. V. (2010). Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World Journal of Gastroenterology, 16 (18), 2202-2222.
24. Mitterdorfer, G., Mayer, H. K., Kneifel, W., Viernstein, H. (2002). Clustering of Saccharomyces boulardii strains within the species S. cerevisiae using molecular typing techniques. Journal of Applied Microbiology, 93, 521–530.
25. Pothoulakis, C. (2009). Review article: anti-inflammatory mechanisms of action of Saccharomyces boulardii. Alimentary Pharmacology & Therapeutics, 30, 826–833.
26. Sindhu, S.C., Khetarpaul, N. (2002). Effect of probiotic fermentation on antinutrients and in vivo protein and starch digestibilities of indigenously developed RWGT food mixture. Nutrition and Health, 16, 173-181.
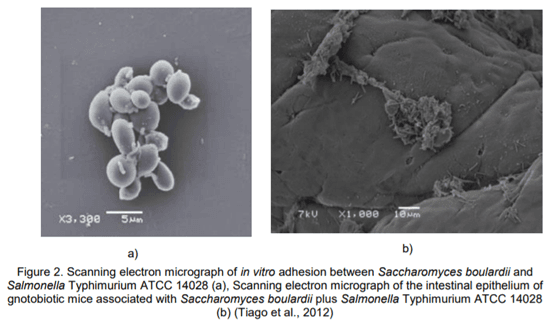
27. Tiago, F.C.P., Martins, F.S., Souza, E.L.S., Pimenta, P.F.P., Araujo, H.R.C., Castro, I.M., Brandao, R.L., Nicoli, J.R. (2012). Adhesion to the yeast cell surface as a mechanism for trapping pathogenic bacteria by Saccharomyces probiotics. Journal of Medical Microbiology, 61, 1194–1207.
28. Tomicic, R., Cabarkapa, I., Vukmirovic, Ð., Levic, J., Tomicic, Z. (2016a). Influence of growth conditions on biofilm formation of Listeria monocitogenes. Food and Feed Research, 43 (1), 19-24.
29. Tomicic, Z., Zupan, J., Matos, T., Raspor, P. (2016b). Probiotic yeast Saccharomyces boulardii (nom. nud.) modulates adhesive properties of Candida glabrata. Medical Mycology, 54 (8), 835-845.
30. van der Aa Kühle, A.,Jespersen, L. (2003). The taxonomic position of Saccharomyces boulardii as evaluated by sequence analysis of the D1/D2 domain of 26S rDNA, the ITS1-5.8S rDNA-ITS2 region and the mitochondrial cytochrome-c oxidase II gene. Systematic and Applied Microbiology, 26 (4), 564-71.
31. van der Aa Kühle, A., Skovgaard, K., Jespersen, L. (2005). In vitro screening of probiotic properties of Saccharomyces cerevisiae var. boulardii and food-borne Saccharomyces cerevisiae strains. International Journal of Food Microbiology, 101, 29–39.
32. Venugopalan, V., Shriner, K.A., Wong-Beringer, A. (2010). Regulatory oversight and safety of probiotic use. Emerging Infectious Diseases, 16, 1661–1665.
33. Qamar, A., Aboudola, S., Warny, M., Michetti, P., Pothoulakis, C., LaMont, J.T., Kelly, C.P. (2001). Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to Clostridium difficile toxin A in mice. Infection and Immunity, 69 (4), 2762–2765.
34. Whelan, K., Myers, C.E. (2010). Safety of probiotics in patients receiving nutritional support: A systematic review of case reports, randomized controlled trials, and nonrandomized trials. The American Journal of Clinical Nutrition, 91, 687–703.
35. Wohlgemuth, S., Gunnar Loh, G., Blaut, M. (2010). Recent developments and perspectives in the investigation of probiotic effects. International Journal of Medical Microbiology, 300, 3–10.








.jpg&w=3840&q=75)