Evaluation of Substituting the Sieving Wastes of the Egyptian Clover's Seeds instead of Soya Bean in the Diet of Flan-Line Rabbits
Published: April 21, 2015
Source : Abdelhamid, A.M. and M.T.M.Saleh (Animal Production Department, Faculty of Agriculture, Al-Mansourah University, Al-Mansourah, Egypt)
Summary
In a feeding trial with rabbits for eight weeks, soya bean (control diet) was substituted by sieving wastes of the Egyptian clover seeds (GSPB, treated diet) to study its possibility, effects and economics for rabbits. This substitution had been succeed; since the treated diet had improved (than the control diet) either of final live body weight of rabbits, daily and total live body weight gain, feed intake and conversion, economic efficiency, boneless meat, and chemical composition of the rabbits' meat without any adverse effect on rabbits' health. So, this substitution is recommended when GSPB is available.
Keywords: Rabbits –Performance - Carcass – Blood – Clover seeds' waste.
INTRODUCTION
Rabbits are neglected farm animals for its smallest size, although it can supplement humans by meat, fur and hair. there animals are belonging to different species and strains which are different in their morphology, color, size, and production purpose. Rabbit's meat contains the highest crude protein content and the lowest fat, cholesterol and energy contents among the livestock animals. Domestic rabbits have a dressing percentage of 50-59 and total edible parts as 78-80%. Rabbits are the best converter of herbs into meat; since in intensive production system, the yearly meat production from the offspring is about 29 times the mother weight. However, rabbits are produced under one of the following systems: extensive, semi-intensive, and intensive production systems (Abdelhamid, 1991). Rabbits are wild mammalian small animals which were known for ancient Egyptians from the 5th Family 2500 originated in Africa and Mediterranean countries and from there outspread all over the world. Rabbits are belonging to the Family: Leporidae and to one origin (Lepuscuniculus). Among their advantages are high prolific, reproductive efficacy, ease feeding and breeding, not expensive production costs, high producing for meat and fur, their meat is easily digestible, long productivity (8 years) and high economic return. One pair could produce 288 – 312 Kg meat / year (Abdellatif, 2008). However, in Egypt, rabbits are raised mainly for meat production but with a modest contribution level. There is no as such a national strategy for rabbit production (Daader, 2005). In Egypt also, there is a huge gap in the animal food; so, the aim of the present study was to evaluate the effects of substitution one of the by-products of the most important artificial forage seeds in Egypt, i.e. Egyptian clover (Berseem) Trifolium alexandrinum instead of soybean meal in rabbits' diet on their growth performance, feed utilization, blood profile, slaughter test and economic evaluation.
MATERIALS AND METHODS
An indoor experimental study was carried out in a private farm for two months during November 2014-January 2015 to evaluate the substitution of ground sieving product of the berseem (GSPB, sieving wastes of the Egyptian clover's (Trifolium alexandrinum seeds)instead of soya bean (on basis of crude protein content) in the diet of Flan rabbits.
Animals and rearing system: Twelve male Spanish V-line rabbits (flesh producing cross) at the age of 25 days [from birth (on the 21st October 2014) until weaning] with a similar average body weight of 500 g were purchased from the Experimental Station of Saba-Basha Faculty of Agriculture, Alexandria University at Sekena village. The rabbits were divided into two groups (each of six rabbits). The animals were housed individually and daily offered weighed feed and measured drinking water ad libitum.
Feeding system: The experimental diet was pelleted to 3.5 mm diameter and 1.5 cm length (because it is a suitable diameter and length for ease rabbits' feeding) after steam cooking at 45 ºC (to protect the ingredients and feed additives from decomposition and/or destruction by heating at higher degrees of temperature, and to obtain pellets less solid and dryness) using a Chinese press in Hemaia Factory for Feed Manufacture in Mansoura. The commercial diet was pelleted in Meet-Ghamr Factory of Feed Manufacture. An anti-coccidian, immune-stimulant and growth promoter (Pep from Biomin, Austria) was added to both diets. Gradually increased quantities of both diets (experimental and control) were offered daily. The daily actual feed and water consumptions were calculated.
Daily observations and management: Daily observations were inspected besides weighing the rabbits, feed and drinking water.
Digestibility trials: At the last 5 days of the fattening experiment (as a collection period), daily feed consumption and feces excreted were weighed, samples were taken and kept in a refrigerator at – 4 ºC till the chemical analysis was undertaken to calculate the digestibility of different nutrients by 3 animals/treatment.
Slaughter test: At the end of the digestibility trials, the same 3 animals/treatment were selected randomly, slaughtered, de-headed, de-skinned, eviscerated, the carcass was divided into different parts and each organ and/or parts was weighed individually. Thereafter, the flesh was fillet and the dressing and boneless meat percentages were calculated.
Blood sampling: During the slaughter test, blood samples were collected from each animal from the jugular vein into vacutainer tubes for hematological and biochemical analyses of blood.
Hematological parameters: Hematological parameters including count of red blood cells (RBC's) and white blood cells (WBC's), packed cell volume (PCV%), and hemoglobin concentration were counted or measured in fresh whole blood using fully digital hematology counter (Laboratories, USA).
Blood serum analysis: Other collected samples were allowed to clot and centrifuged at 3500 rpm for 20 minutes to separate blood serum. Serum was carefully decanted into labeled tubes using serological pipettes and stored at -20 ºC until analysis. Total protein and albumin concentrations were determined using commercial kits according to the Douman et al. (1971). Globulin was calculated by difference. Albumin/globulin ratio was calculated according to the formula: A/G = albumin/globulin. Using commercial kits purchased from bio-Merieux, Laboratory Reagents and Products. Creatinin was estimated in serum by the method of Joffe reaction described by Giorgio (1974) with standard creatinin purchased from Boehringer Mannheim Gmb H-W Germany. Activities of serum transaminases AST and ALT were determined according to Reitman and Frankel (1957) using a colorimetric method via commercial kits. Blood serum was tested also for uric acid, cholesterol, triglycerides, and high density lipoprotein (HDL) concentrations using commercial kits. The low density lipoprotein (LDL) concentration was calculated by subtracting the high density lipoprotein (HDL) concentration and triglycerides concentration (divided by 5) from the total cholesterol concentration [LDL = total cholesterol – HDL – (triglycerides / 5)].
Chemical analysis: Dry matter, crude protein, ether extract, crude fiber and ash of feeds and faces were analyzed according to the methods of A.O.A.C. (1990).
Statistical analysis: The obtained numerical data were statistically analyzed using standard error (SE), coefficient of variance (CV %), and t-test according to Sachs (1976), where SE = standard error of the sample (S) / √ n, CV % = 100 [S / mean (?) ], and t = ( ?1 - ?2 ) / [ √ (S12 / n1) + (S22 / n2) ].
RESULTS AND DISCUSSION
General observations:
The experimental rabbits excreted normal colored (yellow) urine whereas the control rabbits excreted bloody urine. Ground sieving product of berseem (GSPB) inclusion in the experimental diet that naturally contains vitamin K as anti-bleeding agent therefore its presence prevent broking the red blood cells, hence the experimental rabbits' urine was yellow-colored but that of the control rabbits was bloody. The experimental diet led to excrete larger feces' particles than the feces' particles from rabbits fed the control diet.
Experimental diet:
The experimental diet was more suitable for feeding the rabbits than the control diet for the wider (3.5 vs. 3.0 mm) and longer (1.0-1.5 vs. 1.0 cm) experimental pellets as well as the lower cooking temperature (45 ºC vs. 80 ºC) than the control ones. Therefore, the experimental diet was strong flavored, more palatable, consumable and more utilized for its complete usefulness than the control one because of the suitable diameter and length of the experimental pellets as well as it is not strong solid or dry for the lower cooking temperature; so, it was ease for rabbits' feeding, ingestion and mechanical digestion. Hence, the experimental diet was responsible for the increased growth of rabbits than the control.
Contrarily, the control (commercial) diet was very hard and dry to be difficult to be consumed, unpalatable, dusty, with deteriorated components and destructed feed additives. These control pellets were less consumed, require longer time and more effort to be consumed because its fall from the mouth, and therefore disrupt the rabbits that become nervosa. The fine particles in the control diet may cause nasal sensitivity. These fine particles are coming from using half the corn quantity from mid-grade and the other half from the fine grade (which contain dust and toxins and less protein; therefore is undesirable). All these bad characteristics made the control diet less consumable and negatively affected the rabbits' growth and feed utilization.
The following Tables present the proximate analysis (Table 1) and ingredients composition (Table 2) used to formulate the experimental diet. Table 3 presents also chemical composition of the experimental and control diets. The ground sieving wastes of the Egyptian clover's (Trifolium alexandrinum) seeds (GSPB) is a high protein (35.4%) containing ingredient (similar to soya bean but cheaper), therefore it is used in the substituting.. Both, experimental (treated) and control diets were somewhat similar in their chemical analysis (iso-caloric and iso-nitogenous). It is worth noting that the substitution (GSPB in the experimental diet instead of soya bean in the control diet) based on the crude protein content.
Table 1: Proximate analysis (% as fed) of the feed ingredients of the experimental diet.

Table 2: Ingredients composition (kg) of the experimental and control diets.

Table 3: Chemical composition (%) of the experimental and control diets.

Rabbit's performance:
Table 4 shows that after 8-weeks feeding period, the rabbits fed the treated diet (containing GSPB) were heavier and gained more total live body weight gain moreover, their daily body weight gain was significantly (P≤0.01) higher than that of the control rabbits fed soya bean containing diet. Yet, the treated rabbits consumed less feed which resulted in significantly (P≤0.01) better feed conversion ratio and economic efficiency than by the control rabbits. There was no effect of dietary treatment on water intake.
Table 4: Means1± standard errors (and coefficient of variance, CV %) of final live body weight, body weight gain, daily live body weight gain, feed intake (gram / rabbit / 8 weeks), water intake (L / head / 8 weeks), feed conversion ratio, and economic efficiency throughout the eight weeks of the experimental feeding period.

Table 5 shows no significant differences in the nutrients digestibility between both rabbits' groups, except for the dry matter.
Table 5: Means1± standard errors (and coefficient of variance, CV %) of nutrients' digestibility coefficients (%) at the end of the eight weeks of the experimental feeding period.

These better results of growth performance, feed utilization and economic efficiency for the treated rabbits than those of the control rabbits may be due to the manufacture conditions which were reflected on the physical characteristics of both diets as mentioned before.
However, Sadek (2011) calculated the feed intake by rabbits as 114-122 g/h/d, FCR as 2.55-2.75, the nutrient digestibility % as 72 for DM, 74 for organic matter (OM), 64-66 CP, 68-81 EE, 31-38 CF, 90-91 NFE (nitrogen free extract), and the economic efficiency as 244-313%. Moreover, the rabbits consumed feed as 85-94 g / h / d in the study of Abdel-Khalek et al. (2012), who gave percentages of nutrients digestibility by these rabbits as 67-73 for DM, 69-75 OM, 75-81 CP, 62-63 CF, 71-83 EE, and 68-76 NFE, and calculated their economic efficiency as 278-314 %. In addition, Selim et al. (2012) calculated the actual daily feed intake by rabbits as 82.7-86.2 g and FCR as 3.44-4.10. Ragab et al. (2013) also gave the daily feed intake per rabbit as 66.7-72.7 g, FCR 2.87-3.54, and the nutrients digestibility percentage as 69.5-70.5 for DM, 70.8-71.9 OM, 71.2-72.3 CP, 64.7-65.6 CF, 76.9-79.2 EE, and 72.5-73.6 NFE. Abu El-Hamd et al. (2013) also calculated the total DM intake by rabbits as 256-281 g / h / d, and % digestibility as 65.2-67.4 for DM, 67.0-68.2 OM, 64.9-67.8 CP, 43.6-46.2 CF, 64.3-69.0 EE, and 67.3-68.6 NFE.
Slaughter traits:
At the end of the experiment, slaughter traits were carried out to calculate the percentage of different parts and organs of the tested rabbits and to collect meat samples for chemical analysis. Table 6 shows no significant (P≥0.05) difference between treated and control diets for most these traits; yet, heart and bone % were lower (P≤0.05 and 0.001, respectively) but boneless meat % was higher (P≤0.05) for treated rabbits than for the control rabbits. Table 7 presents no significant (P≥0.05) differences in chemical analysis of rabbits lion meat between both rabbits groups. The high dressing and boneless meat percentages are in agreement with the previous data for the better growth performance of treated rabbits.
Table 6: Means1± standard errors (and coefficient of variance, CV %) of slaughter traits (% of rabbit's live body weight) at the end of the eight weeks of the experimental feeding period.

Table 7: Means1± standard errors (and coefficient of variance, CV %) of chemical analysis of the rabbits' lion meat (% fresh weight basis) at the end of the eight weeks of the experimental feeding period.
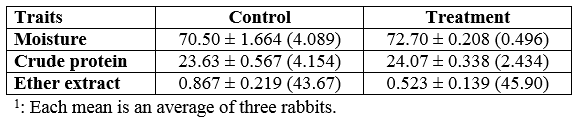
However, Sadek (2011),Abdel-Khalek et al. (2012) and Ragab et al. (2013) calculated the dressing percentage of rabbits as 53.5-55.3, 40.2-42.7, and 50.6-61.6, respectively. Abdel-Khalek et al. (2012) determined the proximate analysis of rabbits' meat and found 70.1-73.0 % CP, 15.3-17.1 % EE, and 11.7-13.5 % ash, on DM basis. Additionally, Selim et al. (2012) calculated dressing percentage of rabbits as 61.3-65.4, liver 2.81-3.53 %, and kidneys 0.70-0.81. Ragab et al. (2013) analyzed rabbit's meat which contained 18.9-21.3 % CP, 1.90-2.79 % EE, and 1.52-1.62 % ash on fresh weight basis. Lastly, El-Medany et al. (2013) calculated the percentages of different carcass' parts of rabbits as 61.6-64.1 for dressing, 15.6-16.4 fore part, 12.0-12.6 middle part, 19.0-20.1 hind part, 10.0-10.3 head, 3.0-3.1 liver, 0.71-0.82 kidneys, 0.32-0.36 heart, and 0.83-0.88 lungs. They (El-Medany et al., 2013) analyzed (% DM basis) also the meat of rabbits as 70.2-79.1 CP, 10.1-18.9 EE, and 10.8-10.9 ash.
Blood picture:
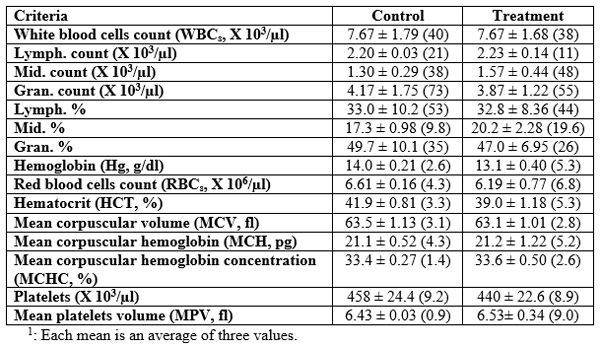
The following Tables (8 and 9) present the hematological and biochemical futures as means ± standard errors (SE) and coefficient of variance (CV %, between brackets) of the experimented rabbits after two months of the feeding period. Table 8 shows no significant (P≥0.05) differences between the treatment and the control for all hematological parameters measured. However, the values of lymphocytes (which are responsible for immunity), mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and mean platelets volume were higher for the treated rabbits than for the control ones; that may reflect the positive effects of the GSPB included diet on the health and hence the performance of the treated rabbits.
Table 8: Means1± standard errors (and coefficient of variance, CV %) of some blood hematological parameters of the rabbits after two months of the experimental feeding.
Yet, the following Table9 shows significantly higher serum glucose (P≤0.01) concentration and ALT (P≤0.05) activity in the control rabbits comparing with the treated rabbits, but there is no significance between the treatment and the control for the other biochemical parameters tested. Generally, treated rabbits had higher values of total proteins, globulin (a future for immunity), and high density lipoprotein, but lower values of glucose, creatinin, uric acid, cholesterol, and low density lipoprotein comparing with the control rabbits confirming the sound life of the treated rabbits. However, the increase in blood sugar is found with hyperactivity of thyroid, pituitary and adrenal glands but the increase in transaminases activity is correlated with the liver disease, where the activity of ALT is higher than the activity of AST (Merck, 1974 and Varley, 1978).
Table 9: Means* ± standard errors (and coefficient of variance, CV %) of some serum biochemical parameters of the rabbits after two months of the experimental feeding.

The low density lipoprotein (LDL, the bad cholesterol) concentration was calculated by subtracting the high density lipoprotein (HDL, the good cholesterol) concentration and triglycerides concentration (divided by 5) from the total cholesterol concentration [LDL = total cholesterol – HDL – (triglycerides / 5)]. However, the CV % among the LDL values was very high because of the scattered values of the individual animals. Its values for the treated rabbits were negative because of the blood samples were collected during the slaughter test without fasting period.
Generally, the obtained values herein for the tested blood parameters are within the normal values given by different authors (Merck, 1976; Abdelhamid, 1988a, b, c; 1989; 1990; Abdelhamid et al., 1999; and Abdelhamid and Saleh, 2000). However, Sadek (2011) gave blood values for rabbits as 5.43-5.88 g / dl total proteins, 3.84-4.47 g / dl albumin, 1.38-1.79 g / dl globulin, 120-129 mg / dl glucose, 30.4-5101 u / l AST, 12.8-14.1 u / l ALT, 0.53-1.05 mg / dl uric acid, 40.7-43.3 mg / dl urea-N, 0.95-1.20 mg / dl creatinin, 171-214 mg / dl cholesterol, 59.3-119 mg / dl triglycerides, 183-338 mg / dl total lipids, 31.7-32.6 mg / dl HDL, and 125-158 mg / dl LDL. Moreover, Abdel-Khalek et al. (2012) gave some blood constituents' values as 7.90-8.52 g / dl total protein, 4.84-5.22 g / dl albumin (A), 3.06-3.33 g / dl globulin (G), 155-1.58 A / G, 72.3-76.5 mg / dl glucose, 0.75-0.82 mg / dl creatinin, 23.2-28.3 u / l AST, 11.9-14.9 u / l ALT, and 1.57-2.38 AST / ALT. Abu El-Hamd et al. (2013) also determined some constituents of rabbits' blood plasma as 7.10-7.96 g / dl total proteins, 3.46-3.73 g / dl albumin, 3.64-4.30 g / dl globulin, 21.4-26.9 mg / dl urea –N, 1.65-1.80 mg / dl creatinin, 41.6-43.6 u / l AST, and 26.3-27.6 u / l ALT. Lastly, El-Medany et al. (2013) registered some blood values for rabbits as 56-76 mg / dl triglyceride, 50-62 mg / dl total cholesterol, 27-34.6 mg / dl HDL, and 12-41.5 mg / dl LDL.
So, there were no negative effects on the experimental rabbits, particularly on the treated ones which fed the GSPB (which is a high protein and cheap agricultural by-product) included diet for eight weeks. Even GSPB prevent occurrence of blood in rabbits' urine as presented in the control rabbits' excreta.
REFERENCES
Abdelhamid, A. M. (1988a). Effect of iron supplementation in rabbits and sheep diets. J. Agric. Sci. Mansoura Univ., 13: 642-650.
Abdelhamid, A. M. (1988b). Effect of dietary contamination with mercury on the performance of rabbits. Archiv für Tierernährung, 38: 207-214.
Abdelhamid, A. M. (1988c). Physionutritional effects of rubratoxin-B on rabbits. Arch. Anim. Nutr., Berlin, 38: 825-832.
Abdelhamid, A. M. (1989). Possibility of fat addition in the rabbit diets. Arch. Anim. Nutr., Berlin, 39: 729-739.
Abdelhamid, A. M. (1990). Effect of feeding rabbits on naturally moulded and mycotoxin-contaminated diet. Arch. Anim. Nutr., Berlin, 40: 55-63.
Abdelhamid, A.M. (1991). Husbandry of Farm, 1st Ed., Dar Al-Nashr for the Egyptian Universities/Al-Waf'a Bookshop, Cairo/ Al-Mansoura, Deposition No.: 7136/1990, ISBN: 977-15-0018-X.
Abdelhamid, A. M. and Saleh, M. R. M. (2000). Effect of graded levels of dietary oxalic acid on growth performance, physiological responses and histological alterations in New Zealand White rabbits. J. Agric. Sci., Mansoura Univ., 25: 4891 – 4903.
Abdelhamid, A. M., El-Nashar, E. M. and Saleh, M. R. M. (1999). Effect of subacute ochratoxicosis- A by rabbits. Proc. 15th Ann. Conf. Egypt. Soc. Toxicol., Alex., Oct. 6-7, Vol. 1, pp: 71-85.
Abdel-Khalek, A.E., Abdelhamid, A.M., Mehrez, A.F. and El-Sawy, I.(2012). Growth performance, digestibility coefficients, blood parameters and carcass traits of rabbits fed biologically treated diets. J. Animal and Poultry Prod., Mansoura Univ., 3 (5): 227-239.
Abdellatif, Kh. O. (2008). A small project for youth for rabbits' rearing, Part 1. Poultry, 202: 74 – 79.
Abu El-Hamd, M. A., Sheteifa, M. A. M. and Ragab, A. A. (2013). Effect of ascorbic acid on performance and reproductive performance of does New Zealand white rabbit. J. Animal and Poultry Prod., Mansoura Univ., 4 (9): 549-559.
A.O.A.C. Association of Official Agricultural Chemists (1990). Official methods of analysis. 15th Ed. Published by the A.O.A.C., Benjamin Francklin Station, Washington. D.C., USA.
Daader, A.H. (2005). Developing rabbit production in Egypt: An overview. Abs. of the 4th Inter. Con. on Rabbit Prod. In Hot Clim. Sharm El-Sheikh, Egypt, P 3.
Douman, B.T.; Waston, W.A. and Homer, G.B. (1971). Albumin standards and the measurement of serum albumin with bromo-cresol green. Clinic. chemical Acta, 31: 87 – 90.
El-Medany, Sh.A.; El-Reffaei, W.H. and Nada, S.A. (2013). Effect of different oils on growth performance and carcass traits in growing rabbits. J. Animal and Poultry Prod., Mansoura Univ., 4 (12): 733-745.
Giorgio, J.D. (1974). Creatinin estimation: Clinical chemistry principles and techniques. Henry et al., eds., pp: 242 – 543 Harper and Row, Hagerstown.
Merck, E. (1974). Klinisches Labor. 12. Auflage, E. Merck, Darmstadt, Deutschland.
Merck, E. (1976). Labordiagnostik in der Tiermedizin. Diagnostica Merck, Deutschland.
Ragab, A.A.; El-Reidy, K.F.A. and Gaafar, H.M.A. (2013). Effect of diet supplementation with pumpkin (Cucurbita moschata) and black seed (Nigella sativa) oils on performance of rabbits: 1- Growth performance, blood hematology and carcass traits of growing rabbits. J. Animal and Poultry Prod., Mansoura Univ., 4 (7): 381-393.
Reitman, S. and Frankel, S. (1957). Colorimetric GOT and GPT Transaminases determination. Amer. J. Clin. Path., 28: 57 – 63.
Sachs, L. (1976). Statistische Methoden, Ein Soforthelfer, Dritte, neubearbeitete Auflage, Springer- Verlag, Berlin.
Sadek, A.M.A. (2011). Studies in feeding rabbits. Ph.D. Thesis, Faculty of Agriculture, Al-Mansourah University.
Selim, N., Abdel-Khalek, A. M. and Gad, S. M. (2012). Effect of supplemental zinc, magnesium or iron on performance and some physiological traits of growing rabbits. Asian Journal of Poultry Science, 6 (1): 23-30.
Varley, H. (1978). Practical Clinical Biochemistry, 4th Ed., Reprinted. Arnold-Heinemann Publishers (India).
Related topics:
Authors:

Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.











