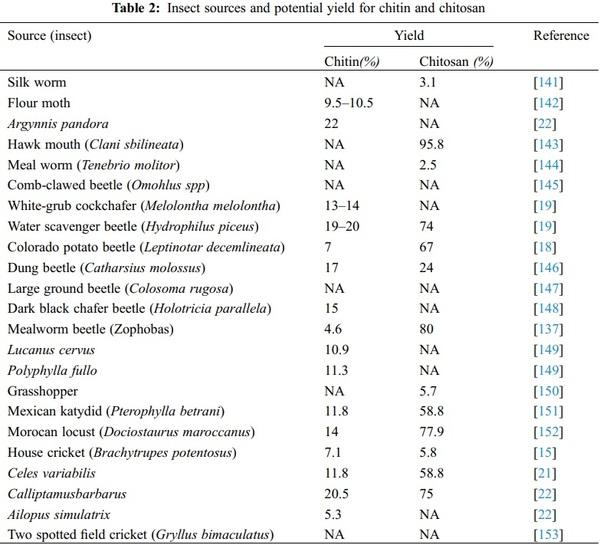
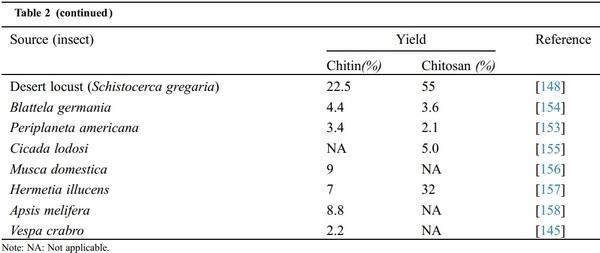
A Review of Various Sources of Chitin and Chitosan in Nature

1. Islam, S., Bhuiyan, M. A. R., Islam, M. N. (2017). Chitin and chitosan: Structure, properties and applications in biomedical engineering. Journal of Polymers and the Environment, 25(3), 854–866. DOI 10.1007/s10924-016-
0865-5.
2. Chhonkar, P. K., Datta, S. P., Joshi, H. C., Pathak, H. (2000). Impact of industrial effluents on soil health and agriculture-Indian experience: Part I–Distillery and paper mill effluents. Journal of Scientific and Industrial
Research, 59(5), 350–361.
3. Gautam, S., Saini, G. (2020). Use of natural coagulants for industrial wastewater treatment. Global Journal of
Environmental Science and Management, 6(4), 553–578. DOI 10.22034/gjesm.2020.04.10.
4. Abo Elsoud, M. M., El Kady, E. M. (2019). Current trends in fungal biosynthesis of chitin and chitosan. Bulletin of the National Research Centre, 43(1), 59. DOI 10.1186/s42269-019-0105-y.
5. Akila, R. M. (2014). Fermentative production of fungal chitosan, a versatile biopolymer (perspectives and its applications). Advances in Applied Science Research, 5(4), 157–170.
6. FAO (2010). Part 1: World review of fisheries and agriculture. The State of World Fisheries and Agriculture,
Marine Policy, 36(3), 746–752. DOI 10.1016/j.marpol.2011.10.021.
7. Ehrlich, H., Shaala, L. A., Youssef, D. T., Żółtowska-Aksamitowska, S., Tsurkan, M. et al. (2018). Discovery of chitin in skeletons of non-verongiid Red Sea demosponges. PLoS One, 13(5), e0195803. DOI 10.1371/journal. pone.0195803.
8. Arbia, W., Arbia, L., Adour, L., Amrane, A. (2013). Chitin extraction from crustacean shells using biological methods–A review. Food Technology and Biotechnology, 51(1), 12–25.
9. Gortari, M. C., Hours, R. A. (2013). Biotechnological processes for chitin recovery out of crustacean waste:
A mini-review. Electronic Journal of Biotechnology, 16(3), 12–25. DOI 10.2225/vol16-issue3-fulltext-10.
10. Ni’mah, Y. L., Harmami, H., Ulfin, I., Suprapto, S., Welny Saleh, C. (2019). Water-soluble chitosan preparation from marine sources. Malaysian Journal of Fundamental and Applied Sciences, 15(2), 159–163. DOI 10.11113/ mjfas.v15n2.971.
11. Rinaudo, M. (2006). Chitin and chitosan: Properties and applications. Progress in Polymer Science, 31(7), 603–
632. DOI 10.1016/j.progpolymsci.2006.06.001.
12. Bhuiyan, M. R., Shaid, A., Bashar, M. M., Haque, P., Hannan, M. A. (2013). A novel approach of dyeing jute fiber with reactive dye after treating with chitosan. Open Journal of Organic Polymer Mater, 3(4), 87–91.
DOI 10.4236/ojopm.2013.34014.
13. Yeul, V. S., Rayalu, S. S. (2013). Unprecedented chitin and chitosan: A chemical overview. Journal of Polymers and the Environment, 21(2), 606–614. DOI 10.1007/s10924-012-0458-x.
14. Sampantamit, T., Ho, L., Lachat, C., Sutummawong, N., Sorgeloos, P. et al. (2020). Aquaculture production and its environmental sustainability in Thailand: Challenges and potential solutions. Sustainability, 12(5), 2010.
DOI 10.3390/su12052010.
15. El Knidri, H., Belaabed, R., Addaou, A., Laajeb, A., Lahsini, A. (2018). Extraction, chemical modification and characterization of chitin and chitosan. International Journal of Biological Macromolecules, 120, 1181–1189.
DOI 10.1016/j.ijbiomac.2018.08.139.
16. Casadidio, C., Peregrina, D. V., Gigliobianco, M. R., Deng, S., Censi, R. et al. (2019). Chitin and chitosans:
Characteristics, eco-friendly processes, and applications in cosmetic science. Marine Drugs, 17(6), 369. DOI
10.3390/md17060369.
17. A., M., Abdel-Rahman, R. M., Hrdina, R., Imramovsky, A., Burgert, L. et al. (2012). Antibacterial cotton fabrics treated with core-shell nanoparticles. International Journal of Biolological Macromolecules, 50(5), 1245–1253.
DOI 10.1016/j.ijbiomac.2012.03.018.
18. Kaya, M., Baran, T., Mentes, A., Asaroglu, M., Sezen, G. et al. (2014). Extraction and characterization of α-chitin and chitosan from six different aquatic invertebrates. Food Biophysics, 9(2), 145–157. DOI 10.1007/s11483-013-
9327-y.
19. Kaya, M., Baublys, V., Can, E., Šatkauskienė, I., Bitim, B. et al. (2014). Comparison of physicochemical properties of chitins isolated from an insect (Melolontha melolontha) and a crustacean species (Oniscus asellus). Zoomorphology, 133(2014), 285–293. DOI 10.1007/s00435-014-0227-6.
20. Hahn, T., Roth, A., Ji, R., Schmitt, E., Zibek, S. (2020). Chitosan production with larval exoskeletons derived from the insect protein production. Journal of Biotechnology, 310, 62–67. DOI 10.1016/j.jbiotec.2019.12.015.
21. Kaya, M., Baublys, V., Satkauskiene, I., Akyuz, B., Bulut, E. et al. (2015). First chitin extraction from Plumatella repens (Bryozoa) with comparison to chitins of insect and fungal origin. International Journal of Biolological
Macromolecules, 79, 126–132. DOI 10.1016/j.ijbiomac.2015.04.066.
22. Kaya, M., Lelesius, E., Nagrockaite, R., Sargin, I., Arslan, G. et al. (2015). Differentiations of chitin content and surface morphologies of chitins extracted from male and female grasshopper species. PLoS One, 10(1), e0115531. DOI 10.1371/journal.pone.0115531.
23. Kaya, M. I., Sargin, K. Ö., Tozak, T., Baran, S., Erdogan, G. S. (2013). Chitin extraction and characterization from Daphnia magna resting eggs. International Journal of Biolological Macromolecules, 61, 459–464. DOI
10.1016/j.ijbiomac.2013.08.016.
24. Klinger, C., Żółtowska-Aksamitowska, S., Wysokowski, M., Tsurkan, M. V., Galli, R. et al. (2019). Express method for isolation of ready-to-use 3D chitin scaffolds from Aplysina archeri (Aplysineidae: Verongiida) demosponge. Marine Drugs, 17(3), 131. DOI 10.3390/md17020131.
25. Shaala, L. A., Asfour, H. Z., Youssef, D. T., Żółtowska-Aksamitowska, S., Wysokowski, M. et al. (2019). New source of 3D chitin scaffolds: The Red Sea demosponge pseudoceratinaarabica (Pseudoceratinidae, Verongiida).
Marine Drugs, 17(2), 92. DOI 10.3390/md17020092.
26. Wysokowski, M., Bazhenov, V. V., Tsurkan, M. V., Galli, R., Stelling, A. L. et al. (2013). Isolation and identification of chitin in three-dimensional skeleton of aplysinafistularis marine sponge. International Journal of Biological Macromolecules, 62, 94–100. DOI 10.1016/j.ijbiomac.2013.08.039.
27. Wang, S. L., Nguyen, V. B. (2019). Production of potent antidiabetic compounds from shrimp head powder via
Paenibacillus conversion. Process Chemistry, 76, 18–24. DOI 10.1016/j.procbio.2018.11.004.
28. Muñoz, I., Rodríguez, C., Gillet, D., Moerschbacher, B. M. (2018). Life cycle assessment of chitosan production in India and Europe. International Journal of Life Cycle Assessment, 23(2018), 1151–1160. DOI 10.1007/ s11367-017-1290-2.
29. Santos, V. P., Marques, N. S. S., Maia, P. C. S. V., Lima, M. A. B., de Franco, L. et al. (2020). Seafood waste as attractive source of chitin and chitosan production and their applications. International Journal of Molecular
Sciences, 21(12), 4290. DOI 10.3390/ijms21124290.
30. Spranghers, T., Ottoboni, M., Klootwijk, C., Ovyn, A., Deboosere, S. et al. (2017). Nutritional composition of black soldier fly (Hermetiaillucens) prepupae reared on different organic waste substrates. Journal of the
Science of Food and Agriculture, 97(8), 2594–2600. DOI 10.1002/jsfa.8081.
31. Hatchett, C. (1799). Experiments and observations on shell and bone. In: Bowyer, W., Nichols, J. (Eds.),
Transactions of the royal society of London, vol. 89, pp. 315–334, London: Royal Society.
32. Odier, A. (1823). Mémoir sur la composition chimique des parties cornées des insectes. Mémoirs de la
Societéd’Histoire Naturelle, 1, 29–42.
33. Knorr, D. (1984). Use of chitinous polymer in food–A challenge for food research and development. Food
Technology, 38(1), 85–97.
34. Kumari, S., Rath, P. K. (2014). Extraction and characterization of chitin and chitosan from (Labeorohit) fish scales. Procedia Materials Science, 6(2014), 482–489. DOI 10.1016/j.mspro.2014.07.062.
35. Ruiz, G. A. M., Corrales, H. F. Z. (2017). Chitosan, chitosan derivatives and their biomedical applications. In:
Shalaby, E. (Eds.), Biological activities and application of marine polysaccharides, pp. 87–106. InTech.
London, UK.
36. Crini, G. (2019). Historical review on chitin and chitosan biopolymers. Environmental Chemistry Letters, 17(4),
1623–1643. DOI 10.1007/s10311-019-00901-0.
37. Ferraro, V., Cruz, I. B., Jorge, R. F., Malcata, F. X., Pintado, M. E. et al. (2010). Valorisation of natural extracts from marine source focused on marine by-products: A review. Food Research International, 43(9), 2221– 2233.
DOI 10.1016/j.foodres.2010.07.034.
38. New, N., Furuike, T., Tamura, H. (2011). Chitosan from aquatic and terrestrial organisms and microorganisms.
Production, properties and applications. In: Johnson, B. M., Berkel, Z. E. (Eds.), Biodegradable materials, pp. 29–50. New York: Nova Science Publishers Inc.
39. Badawy, M., Rabea, E. I. (2017). Chitosan and its modifcations as biologically active compounds in diferent applications. In: Masuell, M., Renard, D. (Eds.), Advances in physicochemical properties of biopolymers, pp. 1–108. Sharjah: Bentham Science Publishers.
40. Bonecco, M. B., Martínez Sáenz, M. G., Bufa, L. M. (2017). Chitosan, from residue to industry. In: Masuell, M.,
Renard, D. (Eds.), Advances in physicochemical properties of biopolymers, pp. 224–256. Sharjah: Bentham e-Books, Bentham Science Publishers.
41. Morin-Crini, N., Lichtfouse, E., Torri, G., Crini, G. (2019). Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper biotechnology and environmental chemistry.
Environmental Chemistry Letters, 17(4), 1667–1692. DOI 10.1007/s10311-019-00904-x.
42. Revathi, M., Saravanan, R., Shanmugam, A. (2012). Production and characterization of chitinase from Vibrio species, a head waste of shrimp metapenaeusdobsonii (Miers, 1878) and chitin of sepiellainermis orbigny,
1848. Advances in Bioscience and Biotechnology, 3(4), 392–397. DOI 10.4236/abb.2012.34056.
43. Chien, R., Yen, M., Mau, J. (2016). Antimicrobial and antitumor activities of chitosan from shiitakestipes, compared to commercial chitosan from crab shells. Carbohydrates Polymers, 138(1), 259–264. DOI 10.1016/ j.carbpol.2015.11.061.
44. Dutta, J., Tripathi, S., Dutta, P. K. (2012). Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: A systematic study needs for food applications. Food Science and Technology International,
18(1), 3–34. DOI 10.1177/1082013211399195.
45. Zargar, V., Asghari, M., Dashti, A. (2015). A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Reviews, 2(3), 204–226. DOI 10.1002/cben.201400025.
46. Yan, N., Chen, X. (2015). Sustainability: Don’t waste seafood waste. Nature, 524(7564), 155–157. DOI 10.1038/
524155a.
47. Berezina, N. (2016). Production and application of chitin. Physical Sciences Reviews, 1(9), 1527. DOI 10.1515/ psr-2016-0048.
48. Sajomsang, W., Gonil, P. (2010). Preparation and characterization of ⊍-chitin from cicada sloughs. Materials
Science and Engineering, 30(3), 357–363. DOI 10.1016/j.msec.2009.11.014.
49. Jang, M. K., Kong, B. G., Jeong, Y. I., Lee, C. H., Nah, J. W. (2004). Physicochemical characterization of
α-chitin, β-chitin, and γ-chitin separated from natural resources. Journal of Polymer Science Part A–Polymer
Chemistry, 42(14), 3423–3432. DOI 10.1002/(ISSN)1099-0518.
50. Muzzarelli, R. A. (2011). Chitin nanostructure in living organisms. In: Gupta, N. (Eds), In: Chitin: Formation and diagenesis, pp. 1–34. Netherland: Dordrecht.
51. Zainol Abidin, N. A., Kormin, F., ZainolAbidin, N. A., Mohamed Anuar, N. A. F., Abu Bakar, M. F. (2020). The potential of insects as alternative sources of chitin: An overview on the chemical method of extraction from various sources. International Journal of Molecular Sciences, 21(14), 4978. DOI 10.3390/ijms21144978.
52. Azuma, K., Ifuku, S., Osaki, T., Okamoto, Y., Minami, S. (2014). Preparation and biomedical applications of chitin and chitosan nanofibers. Journal of Biomedical Nanotechnology, 10(10), 2891–2920. DOI 10.1166/ jbn.2014.1882.
53. Andrew, C. A., Wan, A. C., Tai, B. C. (2013). Chitin—A promising biomaterial for tissue engineering and stem cell technologies. Biotechnology Advances, 31(8), 1776–1785. DOI 10.1016/j.biotechadv.2013.09.007.
54. Jayakumar, R., Prabaharan, M., Nair, S. V., Tamura, H. (2010). Novel chitin and chitosan nanofibers in biomedical applications. Biotechnology Advances, 28(1), 142–150. DOI 10.1016/j.biotechadv.2009.11.001.
55. Shah, P., Jogani, V., Mishra, P., Mishra, A. K., Bagchi, T. et al. (2007). Modulation of gancioclovir intestinal absorption in presence of absorption enhancers. Journal of Pharmaceutical Sciences, 96(10), 2710–2722. DOI
10.1002/jps.20888.
56. Pilai, K. S., Paul, W., Sharma, C. P. (2009). Chitin and chitosan polymers: Chemistry, solublity and fiber formation. Progress in Polymer Science, 34, 641–678. DOI 10.1016/j.progpolymsci.2009.04.001.
57. Merzendorfer, H., Zimoch, L. (2003). Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. Journal of Experimental Biology, 206(24), 4393–4412. DOI 10.1242/jeb.00709.
58. Schmitz, C., Auza, L. G., Koberidze, D., Rasche, S., Fischer, R. et al. (2019). Conversion of chitin to defined chitosan oligomers: Current status and future prospects. Marine Drugs, 17(8), 452. DOI 10.3390/md17080452.
59. Kaur, S., Dhillon, G. S. (2014). The versatile biopolymer chitosan: Potential sources evaluation of extraction methods and applications. Critical Reviews in Microbiology, 40(2), 155–175. DOI 10.3109/1040841X.2013.770385.
60. João, C. F. C., Silva, J. C., Borges, J. P. (2015). Chitin-based nanocomposites: Biomedical applications. In:
Eco-friendly polymer nanocomposites, pp. 439–457. New Delhi: Springer.
61. Ramírez, M. A., Rodriguez, A. T., Alfonso, L., Peniche, C. (2010). Chitin and its derivatives as biopolymers with potential agricultural applications. Biotecnología Aplicada, 27(4), 270–276.
62. Benhabileset, M. S., Salah, R., Lounici, H., Drouiche, N., Goosen, M. F. A. et al. (2012). Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocolloids, 29(1), 48–56. DOI
10.1016/j.foodhyd.2012.02.013.
63. Park, B. K., Kim, M. M. (2010). Applications of chitin and its derivatives in biological medicine. International
Journal of Molecular Sciences, 11(12), 5152–5164. DOI 10.3390/ijms11125152.
64. Fan, W., Yan, W., Xu, Z., Ni, H. (2012). Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids and Surfaces B: Biointerfaces, 90, 21–27. DOI 10.1016/j. colsurfb.2011.09.042.
65. Nowak, V., Persijn, D., Rittenschober, D., Charrondiere, U. R. (2016). Review of food composition data for edible insects. Food Chemistry, 193(2), 39–46. DOI 10.1016/j.foodchem.2014.10.114.
66. Chae, K. S., Shin, C. S., Shin, W. S. (2018). Characteristics of cricket (Gryllus bimaculatus) chitosan and chitosan-based nanoparticles. Food Science and Biotechnology, 27(3), 631–639. DOI 10.1007/s10068-018-
0314-4.
67. Lichtfouse, E., Morin-Crini, N., Fourmentin, M., Zemmouri, H., Oliveira do Carmo Nascimento, I. et al. (2019).
Chitosan for direct bioflocculation processes. In: Crini, G., Lichtfouse, E. (Eds.), Sustainable agriculture reviews, vol. 36, pp. 335–380. Berlin, Germany: Springer International Publishing.
68. Abdel-Rahman, R. M., Hrdina, R., Abdel-Mohsen, A. M., Fouda, M. M. G., Soliman, A. Y. et al. (2015). Chitin and chitosan from Brazilian Atlantic Coast: Isolation, characterization and antibacterial activity. International
Journal of Biological Macromolecules, 80, 107–120. DOI 10.1016/j.ijbiomac.2015.06.027.
69. Elieh-Ali-Komi, D., Hamblin, M. R. (2016). Chitin and chitosan: Production and application of versatile biomedical nanomaterials. International Journal of Advanced Research, 4(3), 411–427.
70. Kumirska, J. M., Czerwicka, Z., Kaczyński, A., Bychowska, K., Brzozowski, J. et al. (2010). Application of spectroscopic methods for structural analysis of chitin and chitosan. Marine Drugs, 8(5), 1567–1636. DOI
10.3390/md8051567.
71. Islam, M. S., Khan, S., Tanaka, M. (2004). Waste loading in shrimp and fish processing effluents: Potential source of hazards to the coastal and nearshore environments. Marine Pollution Bulletine, 49(1–2), 103–110. DOI
10.1016/j.marpolbul.2004.01.018.
72. Rhazi, M., Tolaimate, A., Habibi, Y. (2012). Interactions of chitosan with metals for water purification. In:
Polysaccharide building blocks, pp. 127–141. Hoboken, New Jersey, United States: John Wiley & Sons, Inc.
73. Kumari, S., Kishor, R. (2020). Chitin and chitosan: Origin, properties, and applications. In: Handbook of chitin and chitosan, pp. 1–33. Amsterdam, Netherlands: Elsevier.
74. Rangel-Mendez, J. R., Barrios, V. A. E., Davila-Rodriguez, J. L. (2010). Chitin based biocomposites for removal of contaminants from water: A case study of fluoride adsorption. In: Elnashar, M. (Eds.), Biopolymers. InTech.
London, UK.
75. Younes, I., Rinaudo, M. (2015). Chitin and chitosan preparation from marine sources. Marine Drugs, 13(3),
1133–1174. DOI 10.3390/md13031133.
76. Krajewska, B. (2005). Membrane-based processes performed with use of chitin/chitosan materials. Separation and Purification Technology, 41(3), 305–312. DOI 10.1016/j.seppur.2004.03.019.
77. Inoue, K., Baba, Y. (2007). Chitosan: A versatile biopolymer for separation, purification, and concentration of metal ions. In: Senguta, A. K. (Eds.), Ion exchange and solvent extraction, vol. 18. Boca Raton: CRC Press.
78. Hahn, T., Zibek, S. (2018). Sewage polluted water treatment via chitosan: A review. In: Dongre, R. S. (Eds.),
Chitin-Chitosan—Myriad functionalities in science and technology. InTech. London, UK.
79. Ioelovich, M. (2014). Crystallinity and hydrophility of chitin and chitosan. Research and Reviews: Journal of
Chemistry, 3(3), 7–14.
80. Wang, S. L., Kaoc, T. Y., Wang, Y. H., Yen, Y. H., Chern, T. Y. et al. (2006). Solvent stable metalloprotease produced by Bacillus sp. TKU004 and its application in deproteinization of squid pen for bchitin preparation.
Enzyme and Microbial Technology, 39(4), 724–727. DOI 10.1016/j.enzmictec.2005.12.007.
81. Yang, J. K., Shih, I. L., Tzeng, Y. M., Wang, S. L. (2000). Production and purification of protease from a Bacillus subtilis that can deproteinize crustacean wastes. Enzyme and Microbial Technology, 26(5–6), 406–413. DOI
10.1016/S0141-0229(99)00164-7.
82. Abdou, E. S., Nagy, K. S., Elsabee, M. Z. (2008). Extraction and characterization of chitin and chitosan from local sources. Bioresource Technology, 99(5), 1359–1367. DOI 10.1016/j.biortech.2007.01.051.
83. Di Nardo, T. (2018). Deacetylation by mechanochemistry and aging as a pathway to high molecular weight chitosan from chitin (Masters Thesis). Montreal, Canada: McGill University Canada.
84. Yadav, M., Goswami, P., Paritosh, K., Kumar, M., Pareek, N. et al. (2019). Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresources and Bioprocessing, 6(1),
1–20. DOI 10.1186/s40643-019-0243-y.
85. Zimri, M. N. (2018). Preparation and electrospinning of chitosan from waste black soldier fly biomass (PhD.
Thesis). Bellville, South Africa: University of the Western Cape.
86. Boudouaia, N., Bengharez, Z., Jellali, S. (2019). Preparation and characterization of chitosan extracted from shrimp shells waste and chitosan film: Application for eriochrome black T removal from aqueous solutions.
Applied Water Science, 9(4), 1–12. DOI 10.1007/s13201-019-0967-z.
87. Tokuyasu, K., Mitsutomi, M., Yamaguchi, I., Hayashi, K., Mori, Y. (2000). Recognition of chitooligosaccharides and their N-acetyl groups by putative subsites of chitin deacetylase from a deuteromycete, colletotrichum lindemuthianum. Biochemistry, 39(30), 8837–8843. DOI 10.1021/bi0005355.
88. Philibert, T., Lee, B. H., Fabien, N. (2017). Current status and new perspectives on chitin and chitosan as functional biopolymers. Applied Biochemistry and Biotechnology, 181(4), 1314–1337. DOI 10.1007/s12010-
016-2286-2.
89. Hajji, S., Younes, I., Ghorbel-Bellaaj, O., Hajji, R., Rinaudo, M. et al. (2014). Structural differences between chitin and chitosan extracted from three different marine sources. International Journal of Biological
Macromolecules, 65(4), 298–306. DOI 10.1016/j.ijbiomac.2014.01.045.
90. Aiba, S. I. (1991). Studies on chitosan: Evidence for the presence of random and block copolymer structures in partially N-acetylated chitosans. International Journal of Biological Macromolecules, 13(1), 40–44. DOI
10.1016/0141-8130(91)90008-I.
91. Tacon, A. G. J. (2018). Global trends in aquaculture and compound aquafeed production. Magazine of the World
Aquaculture Society, 49(2), 33–46.
92. Gillett, R. (2008). Global study of shrimp fisheries. Rome: FAO, FAO Fisheries Technical Paper, 331.
93. Kurita, K. (2006). Chitin and chitosan: Functional biopolymers from marine crustaceans. Marine Biotechnology,
8(3), 203–226. DOI 10.1007/s10126-005-0097-5.
94. Pittman, S. J., McAlpine, C. A. (2003). Movements of marine fish and decapod crustaceans: Process, theory and application. Advances in Marine Biology, 44(1), 205–294. DOI 10.1016/S0065-2881(03)44004-2.
95. Abdel-Gawad, K. M., Hifney, A. F., Fawzy, M. A., Gomaa, M. (2017). Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocolloids, 63(2), 593–601.
DOI 10.1016/j.foodhyd.2016.10.001.
96. Turunen, J., Karppinen, A., Ihme, R. (2019). Effectiveness of biopolymer coagulants in agricultural wastewater treatment at two contrasting levels of pollution. SN Applied Sciences, 1(3), 210. DOI 10.1007/s42452-019-0225-x.
97. Pawadee, M., Malinee, P., Thanawit, P., Junya, P. (2003). HetrogeneousNdeacetylation of squid chitin in alkaline solution. Carbohydrate Polymers, 52(2), 119–123. DOI 10.1016/S0144-8617(02)00300-4.
98. Chandumpaia, A., Singhpibulpornb, N., Faroongsarngc, D., Sornprasit, P. (2004). Preparation and physicochemical characterization of chitin and chitosan from the pens of the squid species, loligolessoniana and loligoformosana. Carbohydrate Polymers, 58(4), 467–474. DOI 10.1016/j.carbpol.2004.08.015.
99. Sakthivel, D., Vijayakumar, N., Anandan, V. (2015). Extraction of chitin and chitosan from Mangrove crab (Sesarmaplicatum) from Thengaithittu Estuary Pondicherry Southeast Coast of India. Human Journals, 4(1),
12–24.
100. Ali, M., Shakeel, M., Mehmood, K. (2019). Extraction and characterization of high purity chitosan by rapid and simple techniques from mud crabs taken from Abbottabad. Pakistan Journal of Pharmaceutical Sciences, 32(1),
171–175.
101. Prasetya, D. S. B., Ahzan, S. (2019). Time stirring effect of chitosan from shrimp shells as gold absorbent. Lensa
JurnalKependidikan Fisika, 7(2), 46–50. DOI 10.33394/j-lkf.v7i2.2685.
102. Almeida, L., Rodrigues, W. L., Aguiar, N. V., Silva, R. S., Moreira, C. K. P. (2015). Extração de quitina, síntese e caracterização de quitosanaobtidaatravés de resíduos de camarão (Macrobrachiumamazonicum). Blucher
Chemical Engineering Proceedings, 1(3), 2272–2278. DOI 10.5151/ chemeng-cobeqic2015-477-34107-262119.
103. Santos, V. P., Maia, P., Alencar, N., de, S., Farias, L. et al. (2019). Recovery of chitin and chitosan from shrimp waste with microwave technique and versatile application. Arquivos Do Instituto Biológico, 86(5), 1–7. DOI
10.1590/1808-1657000982018.
104. Souza, F. M., Ferreira, R. M. S., Barbosa, R. C. (2015). Utilização da casca de camarão para produção de quitina.
1–11, Revista Scire.
105. Mahdy Samar, M., El-Kalyoubi, M. H., Khalaf, M. M., Abd El-Razik, M. M. (2013). Physicochemical, functional, antioxidant and antibacterial properties of chitosan extracted from shrimp wastes by microwave technique. Annals of Agricultural Sciences, 58(1), 33–41. DOI 10.1016/j.aoas.2013.01.006.
106. Abdelmalek, B. E., Sila, A., Haddar, A., Bougatef, A., Ali, M. (2017). Chitin and chitosan from squid gladius:
Biological activities of chitosan and its application as clarifying agent for apple juice. International Journal of
Biological Macromolecules, 104, 953–962. DOI 10.1016/j.ijbiomac.2017.06.107.
107. Abdulwadud Abdulkarim, A. O. A., Muhammed, T. I., Surajudeen, A., Abubakar, J. M. (2013). Extraction and characterisation of chitin and chitosan from civ. Environmental Research, 3(2), 108–115.
108. Cumberlidge, N., Daniels, S. R. (2008). A conservation assessment of the freshwater crabs of western Africa (Brachyura: Potamonautidae). African Journal of Ecology, 46(1), 74–79. DOI 10.1111/j.1365-
2028.2007.00815.x.
109. Yeo, D. C. J., Ng, P. K. L., Cumberlidge, N., Magalhaes, C., Daniels, S. R. et al. (2007). Global diversity of crabs (Crustacea: Decapoda: Brachyura) in freshwater. Freshwater Animal Diversity Assessment. Hydrobiologia,
595(2007), 275–286.
110. Fadlaoui, S., Mohammed, L., Omari, A., Melhaoui, M., Sihame, A. et al. (2019). Isolation and characterization of chitin from shells of the freshwater crab potamon algeriense. Progress on Chemistry and Application of Chitin and Its Derivatives, 14, 23–25. DOI 10.15259/PCACD.24.002.
111. Synowiecki, J., Al-Khateeb, N. A. (2003). Production, properties, and some new applications of chitin and its derivatives. Critical Reviews in Food Science and Nutrition, 43(2), 145–171. DOI 10.1080/10408690390826473.
112. Tharanathan, R. N., Kittur, F. S. (2003). Chitin-the undisputed biomolecule of great potential. Critical Reviews in
Food Science and Nutrition, 43(1), 61–87. DOI 10.1080/10408690390826455.
113. Chakrabarti, R. (2002). Carotenoprotein from tropical brown shrimp shell waste by enzymatic process. Food biotechnology, 16(1), 81–90. DOI 10.1081/FBT-120004202.
114. No, H. K., Lee, K. S., Meyers, S. P. (2000). Correlation between physicochemical characteristics and binding capacities of chitosan products. Journal of Food Science, 65(7), 1134–1137. DOI 10.1111/j.1365-2621.2000. tb10252.x.
115. Elasri, O., Salem, M., Ramdani, M., Zaraali, O., Latrach, L. (2018). Effect of increasing inoculum ratio on energy recovery from chicken manure for better use in Egyptian agricultural farms. Chemical and Biological
Technologies in Agriculturure, 17(5), 2–7. DOI 10.1186/s40538-018-0129-9.
116. Cárdenas, G., Cabrera, G., Taboada, E., Miranda, S. P. (2004). Chitin characterization by SEM, FTIR, XRD, and
13C cross polarization/mass angle spinning NMR. Journal of Applied Polymer Science, 93(4), 1876–1885. DOI
10.1002/app.20647.
117. Braconnot, M. H. (1811). LXXXIII. Chemical analysis of the green shell of the walnut. The Philosophical
Magazine, 38(164), 447–451. DOI 10.1080/14786441108638687.
118. Krčmář, M. (2018). Chitosan-glukanovýkomplexizolovanýzeSchizophyllum commune.: The chitosan-glucan complex isolated from Schizophyllum commune (Thesis). Brno-střed, Czechia: Brno University of
Technology. http://hdl.handle.net/11012/4480.
119. Hu, K. J., Yeung, K. W., Ho, K. P., Hu, J. L. (1999). Rapid extraction of high-quality chitosan from mycelia of
Absidia glauca. Journal of Food Biochemistry, 23(2), 187–196. DOI 10.1111/j.1745-4514.1999.tb00013.x.
120. New, N., Chandrkrachang, S., Stevens, W. F., Maw, T., Tan, T. K. et al. (2002). Production of fungal chitosan by solid state and submerged fermentation. Carbohydrates Polymers, 49(2), 235–237. DOI 10.1016/S0144-8617 (01)00355-1.
121. Pochanavanich, P., Suntornsuk, W. (2002). Fungal chitosan production and its characterization. Letters in Applied
Microbiology, 35(1), 17–21. DOI 10.1046/j.1472-765X.2002.01118.x.
122. Suntornsuk, W., Pochanavanich, P., Suntornsuk, L. (2002). Fungal chitosan production on food processing byproducts. Process Biochemistry, 37(7), 727–729. DOI 10.1016/S0032-9592(01)00265-5.
123. Lassaigne, I. L. (1843). Sur les tissues tégumentaires des insectes de differentsordres. Comptes Rendus des
Séances de l’Académie des Sciences, 16, 1087–1089. DOI 10.1007/978-3-030-16538-3_2.
124. Muzzarelli, R. A. A., Boudrant, J., Meyer, D., Manno, N., DeMarchis, M. et al. (2012). Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydrate Polymers, 87(2), 995–
1012. DOI 10.1016/j.carbpol.2011.09.063.
125. Aida, F. M., Shuhaimi, N. A., Yazid, M., Maaruf, A. G. (2009). Mushroom as a potential source of prebiotics:
A review. Trends in Food Science & Technology, 20(11–12), 567–575. DOI 10.1016/j.tifs.2009.07.007.
126. Kalac, P. (2009). Chemical composition and nutritional value of European species of wild growing mushrooms:
A review. Food Chemistry, 113(1), 9–16. DOI 10.1016/j.foodchem.2008.07.077.
127. Jardine, A., Sayed, S. (2018). Valorisation of chitinous biomass for antimicrobial applications. Pure and Applied
Chemistry, 90(2), 293–304. DOI 10.1515/pac-2017-0707.
128. Peter, M. G. (2005). Chitin and chitosan in fungi. In Wiley Biopolymers Online. pp. 123–132. DOI 10.1002/
3527600035.bpol6005.
129. Kim, W. J., Lee, W. G., Theodore, K., Chang, H. N. (2001). Optimization of culture conditions and continuous production of chitosan by the fungi, Absidia coerulea. Biotechnology and Bioprocess Engineering, 6(1), 6–10.
DOI 10.1007/BF02942243.
130. Chatterjee, S., Adhya, M., Guha, A. K., Chatterjee, B. P. (2005). Chitosan from Mucor rouxii: Production and physico-chemical characterization. Process Biochemistry, 40, 395–400. DOI 10.1016/j.procbio.2004.01.025.
131. Sbrana, C., Avio, L., Giovannetti, M. (1995). The occurrence of calcofluor and lectin binding polysaccharides in the outer wall of arbuscular mycorrhizal fungal spores. Mycological Research, 99(10), 1249–1252. DOI 10.1016/
S0953-7562(09)80287-6.
132. Mohan, K., Ganesan, A. R., Muralisankar, T., Jayakumar, R., Sathishkumar, P. et al. (2020). Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends in Food Science &
Technology, 105(4), 17–42. DOI 10.1016/j.tifs.2020.08.016.
133. Gapsari, F., Dewi, F. G., Wijaya, P. H. S., Hidayatullah, S. (2020). The effectiveness of fish scale wastesynthesized chitosan and food-grade chitosan as corrosion inhibitor. Journal of Southwest Jiaotong University,
55(2), 1–10. DOI 10.35741/issn.0258-2724.55.2.47.
134. Dhillon, G. S., Kaur, S., Brar, S. K., Verma, M. (2013). Green synthesis approach: Extraction of chitosan from fungus mycelium. Critical Reviews in Biotechnology, 33(4), 379–403. DOI 10.3109/07388551.2012.717217.
135. Van Huis, A. (2013). Potential of insects as food and feed in assuring food security. Annual Review of
Entomology, 58(1), 563–583. DOI 10.1146/annurev-ento-120811-153704.
136. Shamshina, J. L., Berton, P., Rogers, R. D. (2019). Advances in functional chitin materials: A review. ACS
Sustainable Chemistry & Engineering, 7(7), 6444–6457. DOI 10.1021/acssuschemeng.8b06372.
137. Shin, C. S., Kim, D. Y., Shin, W. S. (2019). Characterization of chitosan extracted from Mealworm beetle (Tenebrio molitor, Zophobasmorio) and Rhinoceros beetle (Allomyrinadichotoma) and their antibacterial activities. International Journal of Biological Macromolecules, 15(125), 72–77.
138. Ibañez-Peinado, D., Ubeda-Manzanaro, M., Martínez, A., Rodrigo, D. (2020). Antimicrobial effect of insect chitosan on Salmonella Typhimurium, Escherichia coli O157: H7 and Listeria monocytogenes survival. PLoS
One, 15(12), e0244153. DOI 10.1371/journal.pone.0244153.
139. Hu, Z. (2018). Challenges and opportunities related to the use of chitosan as food preservative. Journal of Applied
Microbiology, 126(5), 1318–1331. DOI 10.1111/jam.14131.
140. Tolaimate, A., Desbrieres, J., Rhazi, M., Alagui, A. (2003). Contribution to the preparation of chitins and chitosans with controlled physicochemical properties. Polymer, 44(26), 7939–7952. DOI 10.1016/j. polymer.2003.10.025.
141. Battampara, P., Sathish, T. N., Reddy, R., Guna, V., Nagananda, G. S. et al. (2020). Properties of chitin and chitosan extracted from silkworm pupae and egg shells. International Journal of Biological Macromolecules,
15(161), 1296–1304. DOI 10.1016/j.ijbiomac.2020.07.161.
142. Mehranian, M., Pourabad, R. F., Bashir, N. S., Taieban, S. (2017). Physicochemical characterization of chitin from the Mediterranean flour moth, Ephestiakuehniella Zeller (Lepidoptera: Pyralidae). Journal of
Macromolecular Science, Part A, 54(10), 720–726. DOI 10.1080/10601325.2017.1332461.
143. Wu, S. (2012). Preparation of chitooligosaccharides from clanis bilineata larvae skin and their antibacterial activity. International Journal of Biological Macromolecules, 51(5), 1147–1150. DOI 10.1016/j. ijbiomac.2012.08.035.
144. Song, Y. S., Kim, M. W., Moon, C., Seo, D. J., Han, Y. S. et al. (2018). Extraction of chitin and chitosan from larval exuvium and whole body of edible mealworm, Tenebrio molitor. Entomological Research, 48(3), 227–233.
DOI 10.1111/1748-5967.12304.
145. Kaya, M., Mulerčikas, P., Sargin, I., Kazlauskaitė, S., Baublys, V. et al. (2016). Three-dimensional chitin rings from body segments of a pet diplopod species: Characterization and protein interaction studies. Materials
Science and Engineering C, 68, 716–722. DOI 10.1016/j.msec.2016.06.046.
146. Ma, B., Herzog, E. L., Lee, C. G., Peng, X., Lee, C. M. et al. (2015). Role of chitinase 3-like-1 and semaphorin 7a in pulmonary melanoma metastasis. Cancer Research, 75(3), 487–496. DOI 10.1158/0008-5472.CAN-13-3339.
147. Marei, N., Elwahy, A. H., Salah, T. A., El Sherif, Y., Abd El-Samie, E. (2019). Enhanced antibacterial activity of
Egyptian local insects’ chitosan-based nanoparticles loaded with ciprofloxacin-HCl. International Journal of
Biological Macromolecules, 126(12), 262–272. DOI 10.1016/j.ijbiomac.2018.12.204.
148. Liu, X., Cooper, A. M., Yu, Z., Silver, K., Zhang, J. et al. (2019). Progress and prospects of arthropod chitin pathways and structures as targets for pest management. Pesticide Biochemistry and Physiology, 161, 33–46.
DOI 10.1016/j.pestbp.2019.08.002.
149. Kabalak, M., Aracagök, D., Torun, M. (2020). Extraction, characterization and comparison of chitins from large bodied four Coleoptera and Orthoptera species. International Journal of Biological Macromolecules, 145, 402–
409. DOI 10.1016/j.ijbiomac.2019.12.194.
150. Luo, Q., Wang, Y., Han, Q., Ji, L., Zhang, H. et al. (2019). Comparison of the physicochemical, rheological, and morphologic properties of chitosan from four insects. Carbohydrate Polymers, 209(1), 266–275. DOI 10.1016/j. carbpol.2019.01.030.
151. Torres-Castillo, J. A., Sinagawa-García, S. R., Lara-Villalón, M., Martínez-Ávila, G. C. G., Mora-Olivo, A. et al. (2015). Evaluation of biochemical components from Pterophylla beltrani (Bolívar & Bolívar) (Orthoptera:
Tettigoniidae): A forest pest from northeastern Mexico. Southwestern Entomologist, 40(4), 741–751. DOI
10.3958/059.040.0402.
152. Erdogan, S., Kaya, M. (2016). High similarity in physicochemical properties of chitin and chitosan from nymphs and adults of a grasshopper. International Journal of Biological Macromolecules, 89, 118–126. DOI 10.1016/j. ijbiomac.2016.04.059.
153. Ibitoye, E. B., Lokman, I. H., Hezmee, M. N. M., Goh, Y. M., Zuki, A. B. Z. et al. (2018). Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomedical Materials,
13(2), 025009. DOI 10.1088/1748-605X/aa9dde.
154. Kim, Y. H., Park, S. K., Hur, J. Y., Kim, Y. C. (2017). Purification and characterization of a major extracellular chitinase from a biocontrol bacterium, Paenibacilluselgii HOA73. The Plant Pathology Journal, 33(3), 318–328.
DOI 10.5423/PPJ.FT.01.2017.0022.
155. Mol, A., Kaya, M., Mujtaba, M., Akyuz, B. (2018). Extraction of high thermally stable and nanofibrous chitin from cicada (Cicadoidea). Entomological Research, 48(6), 480–489. DOI 10.1111/1748-5967.12299.
156. Caligiani, A., Marseglia, A., Leni, G., Baldassarre, S., Maistrello, L. et al. (2018). Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food
Research International, 105, 812–820. DOI 10.1016/j.foodres.2017.12.012.
157. Khayrova, A., Lopatin, S., Varlamov, V. (2019). Black soldier fly Hermetia illucens as a novel source of chitin and chitosan. International Journal of Sciences, 8(4), 81–86. DOI 10.18483/ijSci.2015.
158. Tsaneva, D., Petkova, Z., Petkova, N., Stoyanova, M., Stoyanova, A. et al. (2018). Isolation and characterization of chitin and biologically active substances from honeybee (Apis mellifera). Journal of Pharmaceutical Sciences and Research, 10(4), 884–888.
159. Bulut, E., Sargin, I., Arslan, O., Odabasi, M., Akyuz, B. et al. (2017). In situ chitin isolation from body parts of a centipede and lysozyme adsorption studies. Materials Science and Engineering: C, 70(2017), 552–563. DOI
10.1016/j.msec.2016.08.048.
160. Jucker, C., Lupi, D., Moore, C. D., Leonardi, M. G., Savoldelli, S. (2020). Nutrient recapture from insect farm waste: Bioconversion with Hermetia illucens (L.) (Diptera: Stratiomyidae). Sustainability, 12(1), 1–14. DOI
10.3390/su12010362.
161. Petrenko, I., Bazhenov, V. V., Galli, R., Wysokowski, M., Fromont, J. et al. (2017). Chitin of poriferan origin and the bioelectrometallurgy of copper/copper oxide. Internatoinal Journal of Biological Macromolecules, 104,
1626–1632. DOI 10.1016/j.ijbiomac.2017.01.084.
162. Greven, H., Kaya, M., Baran, T. (2016). The presence of a-chitin in tardigrada with comments on chitin in the
Ecdysozoa. Journal of Experimental Zoology, 264, 11–16. DOI 10.1016/j.jcz.2016.06.003.
163. Nemtsev, S. V., Zueva, O. Y., Khismatullin, M. R., Albulov, A. I., Varlamov, V. P. (2004). Isolation of chitin and chitosan from Honeybees. Applied Biochemistry and Microbiology, 40, 39–43. DOI 10.1023/B:
ABIM.0000010349.62620.49.
164. Zhang, M., Haga, A., Sekiguchi, H., Hirano, S. (2000). Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Internatoinal Journal of Biological Macromolecules, 27(1), 99–105.
DOI 10.1016/S0141-8130(99)00123-3.
165. Basseri, H., Bakhtiyari, R., Hashemi, S. J., Baniardelani, M., Shahraki, H. et al. (2019). Antibacterial/antifungal activity of extracted chitosan from American cockroach (Dictyoptera: Blattidae) and German cockroach (Blattodea: Blattellidae). Journal of Medical Entomology, 56(5), 1208–1214. DOI 10.1093/jme/tjz082.
166. Majtán, J., Bíliková, K., Markovič, O., Gróf, J., Kogan, G. et al (2007). Isolation and characterization of chitin from bumblebee (Bombus terrestris). International Journal of Biological Macromolecules, 40(3), 237–241. DOI
10.1016/j.ijbiomac.2006.07.010.
167. Finke, M. D. (2013). Complete nutrient content of four species of feeder insects. Zoo Biology, 32(1), 27–36. DOI
10.1002/zoo.21012.
168. Huet, G., Hadad, C., Husson, E., Laclef, S., Lambertyn, V. et al. (2020). Straightforward extraction and selective bioconversion of high purity chitin from bombyx eri larva: Toward an integrated insect biorefinery. Carbohydrate
Polymers, 228, 115382. DOI 10.1016/j.carbpol.2019.115382.
169. Marei, N. H., Abd El-Samie, E., Salah, T., Saad, G. R., Elwahy, A. H. (2016). Isolation and characterization of chitosan from different local insects in Egypt. International Journal of Biological Macromolecules, 82(7), 871–
877. DOI 10.1016/j.ijbiomac.2015.10.024.
170. Henry, M., Gasco, L., Piccolo, G., Fountoulaki, E. (2015). Review on the use of insects in the diet of farmed fish:
Past and future. Animal Feed Science and Technology, 203, 1–22. DOI 10.1016/j.anifeedsci.2015.03.001.
171. FAO (2012). Expert consultation; assesses the potential of insects as food and feed in assuring food security.
Technical Consultation Meeting, pp. 23–25. Rome, Italy.
172. Hanboonsong, Y., Jamjanya, T., Durst, P. B. (2013). Six-legged livestock: Edible insect farming, collection and marketing in Thailand. RAP Publication, 3, 8–21.
173. Kaya, M., Mujtaba, M., Ehrlich, H., Salaberria, A. M., Baran, T. et al. (2017). On chemistry of γ-chitin.
Carbohydrate Polymers, 176(5), 177–186. DOI 10.1016/j.carbpol.2017.08.076.
174. Liu, S., Sun, J., Yu, L., Zhang, C., Bi, J. et al. (2012). Extraction and characterization of chitin from the beetle holotrichia parallela motschulsky. Molecules, 17(4), 4604–4611. DOI 10.3390/molecules17044604.
175. Badariotti, F., Thuau, R., Lelong, C., Dubos, M. P., Favrel, P. (2007). Characterization of an atypical family
18 chitinase from the oyster crassostrea gigas: Evidence for a role in early development and immunity.
Developmental & Comparative Immunology, 31(6), 559–570. DOI 10.1016/j.dci.2006.09.002.
176. Rumpold, B. A., Schlüter, O. K. (2013). Nutritional composition and safety aspects of edible insects. Molecular
Nutrition & Food Research, 57(5), 802–823. DOI 10.1002/mnfr.201200735.
177. Latgé, J. P. (2007). The cell wall: A carbohydrate armour for the fungal cell. Molecular Microbiology, 66(2), 279–
290. DOI 10.1111/j.1365-2958.2007.05872.x.
178. Berger, R., Christina, T., de Miranda, A., Pessoa, P., Barbosa, M. A. et al. (2017). Chitosan produced from mucorales fungi using agroindustrial by-products and its efficacy to inhibit colletotrichum species. International Journal of Biological Macromolecules, 108(2017), 635–641. DOI 10.1016/j. ijbiomac.2017.11.178.
179. Żółtowska-Aksamitowska, S., Shaala, L. A., Youssef, D. T., Elhady, S. S., Tsurkan, M. V. et al. (2018). First report on chitin in a non-verongiid marine demosponge: The mycale euplectellioides case. Marine Drugs,
16(2), 68. DOI 10.3390/md16020068.
180. Croisier, F., Jérôme, C. (2013). Chitosan-based biomaterials for tissue engineering. European Polymer Journal,
49(4), 780–792. DOI 10.1016/j.eurpolymj.2012.12.009.
181. Khor, E., Wu, H., Lim, L. Y., Guo, C. M. (2011). Chitin-methacrylate: Preparation, characterization and hydrogel formation. Materials, 4(10), 1728–1746. DOI 10.3390/ma4101728.










