Radiation Use Efficiency of Forage Resources: A Meta-Analysis
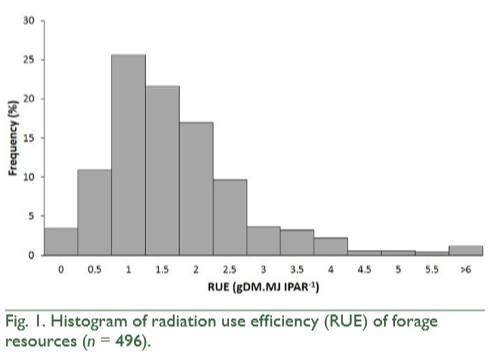
Forage production is increasingly monitored through models based on remote sensing. Radiation use efficiency (RUE) is a key input to these models. However, no study has synthesized the published values of RUE of forage resources. Our objective was to quantitatively synthesize through a meta-analysis the variation of RUE of forage resources and its main controls. We gathered 496 RUE values and assessed their variation according to genotype, resource availability and phenological stage. Mean RUE was 1.93 ± 1.2 g of dry matter (DM) per megajoule of intercepted photosynthetically active radiation (IPAR) (±SD). The RUE was most responsive to genotype and to direct and indirect manipulations of resource availability. Within genotype treatments, mean effect size of C4 species identity, cultivar identity and C3 species identity was 61, 58, and 42%, respectively. Within resource availability treatments, mean effect size was 43% for N fertilization, 40% for shading and inter-annual environmental variation and 23% for irrigation and defoliation frequency. For the phenology treatment, mean effect size of reproductive vs. vegetative stage did not differ from zero. This large variability implies a challenge to select RUE values as input to estimate productivity through plant-growth models, such as those based on remote sensing, but also highlight the margin for increasing RUE through breeding and management practices.
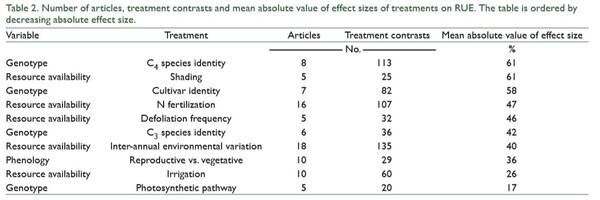
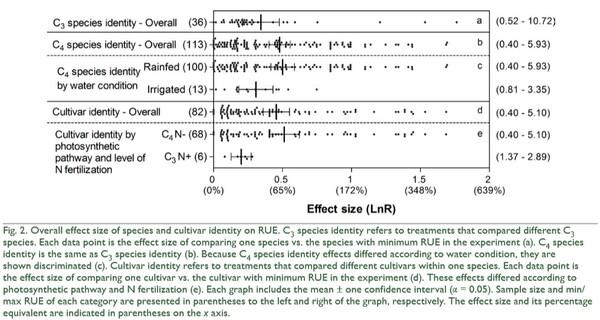
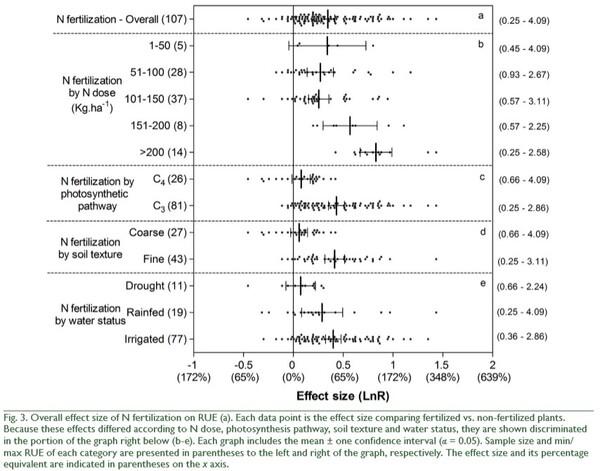
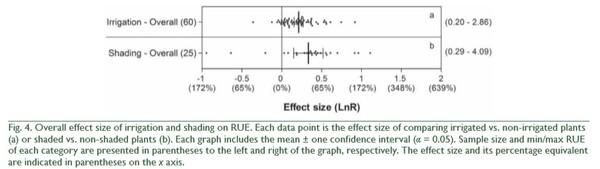
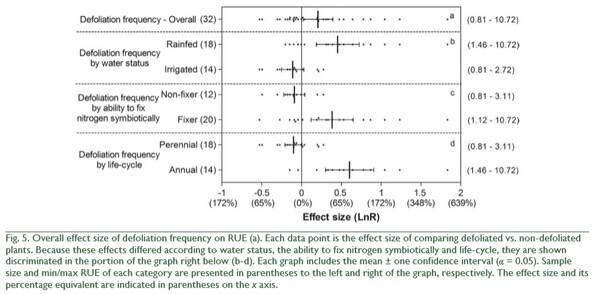
increased more by N fertilization on fine than on coarse textured soils (QB = 25, p < 0.0001; Fig. 3). Finally, the effect of N fertilization on RUE increased as water condition of the experiments became more favorable for plant growth (QB = 10, p = 0.0056; Fig. 3). In addition to these N fertilization effects, irrigation and shading affected RUE (Fig. 4). On average, irrigation increased RUE by 23% and shading increased it by 40% (Fig. 4). In both cases, total heterogeneity was significant among effect sizes (QT = 150, p < 0.0001 and QT = 322, p < 0.0001, respectively). RUE also widely responded to indirect manipulations of resource availability. The mean variation between the RUE of a year and the minimum RUE in the period under study was 40%, with no heterogeneity among effect sizes (QT = 151, p = 0.1538). Higher defoliation frequency increased RUE by 23% (Fig. 5), with significant heterogeneity among effect sizes (QT = 47, p = 0.0324). Defoliation frequency increased RUE under rainfed conditions and did not affect it under irrigation conditions (Fig. 5) (QB = 18, p < 0.0001). Similarly, the positive effect of defoliation frequency was observed in plants with the ability to fix nitrogen symbiotically (QB = 8, p = 0.0049) and in annuals more than in perennials (QB = 32, p < 0.0001; Fig. 5).
Albrizio, R., and P. Steduto. 2005. Resource use efficiency of fieldgrown sunflower, sorghum, wheat and chickpea: I. Radiation use efficiency. Agric. For. Meteorol. 130:254–268. doi:10.1016/j. agrformet.2005.03.009 Alexandrino, E., J.A. Gomide, and C.A.M. Gomide. 2005. Crescimento e desenvolvimento do dossel de Panicum maximum cultivar Mombaça. Rev. Bras. Zootec. 34:2164–2173. doi:10.1590/ S1516-35982005000700002 Avice, J.C., G. Lemaire, A. Ourry, and J. Boucaud. 1997. Effects of the previous shoot removal frequency on subsequent shoot regrowth in two Medicago sativa L. cultivars. Plant Soil 188:189–198. doi:10.1023/A:1004291801023 Bat-Oyun, T., M. Shinoda, and M. Tsubo. 2011. Effects of water and temperature stresses on radiation use efficiency in a semi-arid grassland. J. Plant Interact. 7:214–224. doi:10.1080/17429145.2011.564736 Bélanger, G., F. Gastal, and F.R. Warembourg. 1994. Carbon balance of tall fescue (Festuca arundinacea Schreb.): Effects of nitrogen fertilization and the growing season. Ann. Bot. (Lond.) 74:653–659. doi:10.1006/anbo.1994.1167
Campbell, C.S., J.L. Heilman, K.J. McInnes, L.T. Wilson, J.C. Medley, G. Wu, and D.R. Cobos. 2001. Seasonal variation in radiation use efficiency of irrigated rice. Agric. For. Meteorol. 110:45–54. doi:10.1016/S0168-1923(01)00277-5 Cattivelli, L., F. Rizza, F.-W. Badeck, E. Mazzucotelli, A.M. Mastrangelo, E. Francia, C. Marè, A. Tondelli, and A.M. Stanca. 2008. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crops Res. 105:1–14. doi:10.1016/j. fcr.2007.07.004 Cornic, G., and A. Massacci. 1996. Leaf photosynthesis under drought stress. In: N.R. Baker, editor, Photosynthesis and the environment. Kluwer Academic Publishers, The Netherlands. p. 347–366. Cristiano, P.M., G. Posse, C.M. Di Bella, and T. Boca. 2012. Influence of contrasting availabilities of water and nutrients on the radiation use efficiency in C3 and C4 grasses. Austral Ecol. 37:323–329. doi:10.1111/j.1442-9993.2011.02296.x
Cruz, P. 1996. Growth and nitrogen nutrition of a Dichanthium aristatum pasture under shading. Trop. Grassl. 30:407–413. Cruz, P. 1997. Effect of shade on the carbon and nitrogen allocation in a perennial tropical grass, Dichanthium aristatum. J. Exp. Bot. 48:15– 24. doi:10.1093/jxb/48.1.15 Chaves, M.M., J. Flexas, and C. Pinheiro. 2009. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. (Lond.) 103:551–560. doi:10.1093/aob/mcn125 de Dorlodot, S., B. Forster, L. Pagès, A. Price, R. Tuberosa, and X. Draye. 2007. Root system architecture: Opportunities and constraints for genetic improvement of crops. Trends Plant Sci. 12:474–481. doi:10.1016/j.tplants.2007.08.012
Deng, Z.-N., Y.-W. Wei, W.-L. Lü, and Y.-R. Li. 2008. Fusion insect-resistant gene mediated by matrix attachment region (MAR) sequence in transgenic sugarcane. Sugar Tech 10:87–90. doi:10.1007/ s12355-008-0015-z Earl, H.J., and R.F. Davis. 2003. Effect of drought stress on leaf and whole canopy radiation use efficiency and yield of maize. Agron. J. 95:688– 696. doi:10.2134/agronj2003.0688 Fedorov, S. 2013. GetData Graph Digitizer version 2.26. http://getdatagraph-digitizer.com. Fletcher, A., P. Johnstone, E. Chakwizira, and H. Brown. 2013. Radiation capture and radiation use efficiency in response to N supply for crop species with contrasting canopies. Field Crops Res. 150:126–134. doi:10.1016/j.fcr.2013.06.014
Flexas, J., A. Diaz-Espejo, J. Galmés, R. Kaldenhoff, H. Medrano, and M. Ribas-Carbo. 2007. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ. 30:1284–1298. doi:10.1111/j.1365-3040.2007.01700.x Friesen, D.K., I.M. Rao, R.J. Thomas, A. Oberson, and J.I. Sanz. 1997. Phosphorus acquisition and cycling in crop and pasture systems in low fertility tropical soils. Plant Soil 196:289–294. doi:10.1023/A:1004226708485 Fritz, S., L. See, J.C.L. Bayas, F. Waldner, D. Jacques, I. Becker-Reshef, A. Whitcraft, B. Baruth, R. Bonifacio, J. Crutchfield, F. Rembold, O. Rojas, A. Schucknecht, M. Van der Velde, J. Verdin, B. Wu, N. Yan, L. You, S. Gilliams, S. Mücher, R. Tetrault, I. Moorthy, and I. McCallum. 2019. A comparison of global agricultural monitoring systems and current gaps. Agric. Syst. 168:258–272. doi:10.1016/j. agsy.2018.05.010
Gifford, R.M. 2003. Plant respiration in productivity models: Conceptualization, representation and issues for global terrestrial carbon-cycle research. Funct. Plant Biol. 30:171–186. doi:10.1071/FP02083 Gómez, S., O. Guenni, and L. Bravo de Guenni. 2012. Growth, leaf photosynthesis and canopy light use efficiency under differing irradiance and soil N supplies in the forage grass Brachiaria decumbens Stapf. Grass Forage Sci. 68:395–407. doi:10.1111/gfs.12002
Gosse, G., C. Varlet-Grancher, R. Bonhomme, M. Chartier, J.M. Allirand, and G. Lemaire. 1986. Production maximale de matière sèche et rayonnement solaire intercepté par un couvert végétal. Agronomie 6:47–56. doi:10.1051/agro:19860103 Grigera, G., M. Oesterheld, and F. Pacín. 2007. Monitoring forage production for farmers’ decision making. Agric. Syst. 94:637–648. doi:10.1016/j.agsy.2007.01.001 Guido, A., R.D. Varela, P. Baldassini, and J.M. Paruelo. 2014. Spatial and temporal variability in aboveground net primary production of Uruguayan grasslands. Rangeland Ecol. Manag. 67:30–38. doi:10.2111/ REM-D-12-00125.1 Gurevitch, J., L.L. Morrow, A. Wallace, and J.S. Walsh. 1992. A metaanalysis of competition in field experiments. Am. Nat. 140:539–572. doi:10.1086/285428
Hall, A.J., D.J. Connor, and V.O. Sadras. 1995. Radiation-use efficiency of sunflower crops: Effects of specific leaf nitrogen and ontogeny. Field Crops Res. 41:65–77. doi:10.1016/0378-4290(94)00108-O Hammer, G.L., E. van Oosterom, G. McLean, S.C. Chapman, I. Broad, P. Harland, and R.C. Muchow. 2010. Adapting APSIM to model the physiology and genetics of complex adaptive traits in field crops. J. Exp. Bot. 61:2185–2202. doi:10.1093/jxb/erq095 Harrison, M.T., W.M. Kelman, A.D. Moore, and J.R. Evans. 2010. Grazing winter wheat relieves plant water stress and transiently enhances photosynthesis. Funct. Plant Biol. 37:726–736. doi:10.1071/ FP10040 Haynes, R.J., and G.S. Francis. 1993. Changes in microbial biomass C, soil carbohydrate composition and aggregate stability induced by growth of selected crop and forage species under field conditions. J. Soil Sci. 44:665–675. doi:10.1111/j.1365-2389.1993.tb02331.x Healey, K.D., G.L. Hammer, K.G. Rickert, and M.P. Bange. 1998. Radiation use efficiency increases when the diffuse component of incident radiation is enhanced under shade. Aust. J. Agric. Res. 49:665–672. doi:10.1071/A97100
Hedges, L.V., J. Gurevitch, and P.S. Curtis. 1999. The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156. doi:10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2 Huete, A., K. Didan, T. Miura, E.P. Rodriguez, X. Gao, and L.G. Ferreira. 2002. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 83:195–213. doi:10.1016/S0034-4257(02)00096-2 Irisarri, J.G.N., M. Oesterheld, R.A. Golluscio, and J.M. Paruelo. 2014. Effects of animal husbandry on secondary production and trophic efficiency at a regional scale. Ecosystems 17:738–749. doi:10.1007/ s10021-014-9756-6 Jamieson, P.D., R.J. Martin, G.S. Francis, and D.R. Wilson. 1995. Drought effects on biomass production and radiation-use efficiency in barley. Field Crops Res. 43:77–86. doi:10.1016/0378-4290(95)00042-O Kant, S., Y.-M. Bi, and S.J. Rothstein. 2010. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 62:1499–1509. doi:10.1093/jxb/erq297
Kiniry, J.R., M.-V.V. Johnson, S.B. Bruckerhoff, J.U. Kaiser, R.L. Cordsiemon, and R.D. Harmel. 2012. Clash of the Titans: Comparing productivity via radiation use efficiency for two grass giants of the biofuel field. BioEnergy Res. 5:41–48. doi:10.1007/ s12155-011-9116-8 Kiniry, J.R., L.C. Anderson, M.V.V. Johnson, K.D. Behrman, M. Brakie, D. Burner, R.L. Cordsiemon, P.A. Fay, F.B. Fritschi, J.H. Houx, C. Hawkes, T. Juenger, J. Kaiser, T.H. Keitt, J. Lloyd-Reilley, S. Maher, R. Raper, A. Scott, A. Shadow, C. West, Y. Wu, and L. Zibilske. 2013. Perennial biomass grasses and the Mason-Dixon Line: Comparative productivity across latitudes in the Southern Great Plains. BioEnergy Res. 6:276–291. doi:10.1007/s12155-012-9254-7 Kobe, R.K., M. Iyer, and M.B. Walters. 2010. Optimal partitioning theory revisited: Nonstructural carbohydrates dominate root mass responses to nitrogen. Ecology 91:166–179. doi:10.1890/09-0027.1
Koricheva, J., J. Gurevitch, and L. Gómez Aparicio. 2014. Uses and misuses of meta-analysis in plant ecology. J. Ecol. 102:828–844. doi:10.1111/1365-2745.12224 Kumar, M., and J.L. Monteith. 1982. Remote sensing of plant growth. In: H. Smith, editor, Plants and the daylight spectrum. Academic Press, London. p. 133–144. Lestienne, F., B. Thornton, and F. Gastal. 2006. Impact of defoliation intensity and frequency on N uptake and mobilization in Lolium perenne. J. Exp. Bot. 57:997–1006. doi:10.1093/jxb/erj085 Leys, C., C. Ley, O. Klein, P. Bernard, and L. Licata. 2013. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 49:764–766. doi:10.1016/j.jesp.2013.03.013 Manderscheid, R., M. Erbs, and H.-J. Weigel. 2014. Interactive effects of free-air CO2 enrichment and drought stress on maize growth. Eur. J. Agron. 52:11–21. doi:10.1016/j.eja.2011.12.007 McCall, D.G., and G.J. Bishop-Hurley. 2003. A pasture growth model for use in a whole-farm dairy production model. Agric. Syst. 76:1183– 1205. doi:10.1016/S0308-521X(02)00104-X
Mills, A., D.J. Moot, and P.D. Jamieson. 2009. Quantifying the effect of nitrogen on productivity of cocksfoot (Dactylis glomerata L.) pastures. Eur. J. Agron. 30:63–69. doi:10.1016/j.eja.2008.07.008 Monteith, J.L. 1972. Solar radiation and productivity in tropical ecosystems. J. Appl. Ecol. 9:747–766. doi:10.2307/2401901 Monteith, J.L. 1978. Reassessment of maximum growth rates for C3 and C4 crops. Exp. Agric. 14:1–5. doi:10.1017/S0014479700008255 Muchow, R.C., and T.R. Sinclair. 1994. Nitrogen response of leaf photosynthesis and canopy radiation use efficiency in field-grown maize and sorghum. Crop Sci. 34:721–727. doi:10.2135/cropsci1994.001 1183X003400030022x Muchow, R.C., M.J. Robertson, and B.C. Pengelly. 1993. Radiationuse efficiency of soybean, mugbean and cowpea under different environmental conditions. Field Crops Res. 32:1–16. doi:10.1016/0378-4290(93)90017-H
Nouvellon, Y., D.L. Seen, S. Rambal, A. Bégué, M.S. Moran, Y. Kerr, and J. Qi. 2000. Time course of radiation use efficiency in a shortgrass ecosystem: Consequences for remotely sensed estimation of primary production. Remote Sens. Environ. 71:43–55. doi:10.1016/ S0034-4257(99)00063-2 Pacín, F., and M. Oesterheld. 2015. Closing the technological gap of animal and crop production through technical assistance. Agric. Syst. 137:101–107. doi:10.1016/j.agsy.2015.04.007 Paruelo, J.M., M. Oesterheld, C.M. Di Bella, M. Arzadum, J. Lafontaine, M. Cahuepé, and C.M. Rebella. 2000. Estimation of primary production of subhumid rangelands from remote sensing data. Appl. Veg. Sci. 3:189–195. doi:10.2307/1478997 Piñeiro, G., M. Oesterheld, and J.M. Paruelo. 2006. Seasonal variation in aboveground production and radiation-use efficiency of temperate rangelands estimated through remote sensing. Ecosystems 9:357– 373. doi:10.1007/s10021-005-0013-x Rahman, M.M., D.W. Lamb, J.N. Stanley, and M.G. Trotter. 2014. Use of proximal sensors to evaluate at the sub-paddock scale a pasture growth-rate model based on light-use efficiency. Crop Pasture Sci. 65:400–409. doi:10.1071/CP14071
Roderick, M.L., G.D. Farquhar, S.L. Berry, and I.R. Noble. 2001. On the direct effect of clouds and atmospheric particles on the productivity and structure of vegetation. Oecologia 129:21–30. doi:10.1007/ s004420100760 Rosenberg, M.S., D.C. Adams, and J. Gurevitch. 2000. MetaWin. Statistical software for meta-analysis. Sinauer Assoc., Inc., Sunderland, MA. Rosenberg, M.S., H.R. Rothstein, and J. Gurevitch. 2013. Effect sizes: Conventional choices and calculations. In: J. Koricheva, et al., editors, Handbook of meta-analysis in ecology and evolution. Princeton Univ. Press, Princeton, NJ. p. 61–71. Running, S.W., R.R. Nemani, F.A. Heinsch, M. Zhao, M. Reeves, and H. Hashimoto. 2004. A continuous satellite-derived measure of global terrestrial primary production. Bioscience 54:547–560. doi:10.1641/0006-3568(2004)054[0547:ACSMOG]2.0.CO;2
Sala, O.E., and A.T. Austin. 2000. Methods of estimating aboveground net primary productivity. In: O.E. Sala, et al., editors, Methods in ecosystem science. Springer, New York. p. 31–43. doi:10.1007/978-1-4612-1224-9_3 Sinclair, T.R., and T. Horie. 1989. Leaf nitrogen, photosynthesis, and crop radiation use efficiency: A review. Crop Sci. 29:90–98. doi:10.2135/ cropsci1989.0011183X002900010023x Sinclair, T.R., and R.C. Muchow. 1999. Radiation use efficiency. Adv. Agron. 65:215–265. doi:10.1016/S0065-2113(08)60914-1 Sinclair, T.R., T. Shiraiwa, and G.L. Hammer. 1992. Variation in crop radiation-use efficiency with increased diffuse-radiation. Crop Sci. 32:1281–1284. doi:10.2135/cropsci1992.0011183X0032000500 43x
Slafer, G.A., J.L. Araus, C. Royo, and L.F. García del Moral. 2005. Promising eco-physiological traits for genetic improvement of cereal yields in Mediterranean environments. Ann. Appl. Biol. 146:61–70. doi:10.1111/j.1744-7348.2005.04048.x Slattery, R.A., E.A. Ainsworth, and D.R. Ort. 2013. A meta-analysis of responses of canopy photosynthetic conversion efficiency to environmental factors reveals major causes of yield gap. J. Exp. Bot. 64:3723– 3733. doi:10.1093/jxb/ert207 Stockle, C.O., and J.R. Kiniry. 1990. Variability in crop radiation-use efficiency associated with vapor-pressure deficit. Field Crops Res. 25:171–181. doi:10.1016/0378-4290(90)90001-R Strullu, L., S. Cadoux, N. Beaudoin, and M.H. Jeuffroy. 2013. Influence of belowground nitrogen stocks on light interception and conversion of Miscanthus x giganteus. Eur. J. Agron. 47:1–10. doi:10.1016/j. eja.2013.01.003
Teixeira, E.I., D.J. Moot, and H.E. Brown. 2008. Defoliation frequency and season affected radiation use efficiency and dry matter partitioning to roots of lucerne (Medicago sativa L.) crops. Eur. J. Agron. 28:103–111. doi:10.1016/j.eja.2007.05.004 Varella, A.C., D.J. Moot, K.M. Pollock, P.L. Peri, and R.J. Lucas. 2011. Do light and alfalfa responses to cloth and slatted shade represent those measured under an agroforestry system? Agrofor. Syst. 81:157–173. doi:10.1007/s10457-010-9319-6 Verón, S.R., M. Oesterheld, and J.M. Paruelo. 2005. Production as a function of resource availability: Slopes and efficiencies are different. J. Veg. Sci. 16:351–354. doi:10.1111/j.1654-1103.2005.tb02373.x Wilson, J.R., and M.M. Ludlow. 1991. The environment and potential growth of herbage under plantations. In: H.M. Shelton, and W.W. Stur, editors, Forage for plantation crops. Vol. 32. ACIAR Proceedings, Canberra. p. 10–24.







.jpg&w=3840&q=75)