Heat damage, Maillard reactions, and measurement of reactive lysine in feed ingredients and diets
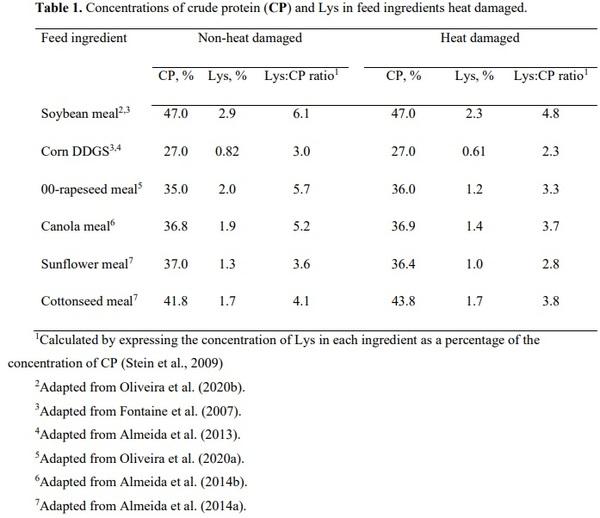
Almeida, F. N., J. K. Htoo, J. Thomson, and H. H. Stein. 2013. Amino acid digestibility of heat damaged distillers dried grains with solubles fed to pigs. J. Anim. Sci. Biotechnol. 4:44-44. doi 10.1186/2049-1891-4-44
Almeida, F. N., J. K. Htoo, J. Thomson, and H. H. Stein. 2014a. Digestibility by growing pigs of amino acids in heat-damaged sunflower meal and cottonseed meal. J. Anim. Sci. 92:585-
593. doi 10.2527/jas.2013-6769
Almeida, F. N., J. K. Htoo, J. Thomson, and H. H. Stein. 2014b. Effects of heat treatment on the apparent and standardized ileal digestibility of amino acids in canola meal fed to growing pigs. Anim. Feed Sci. Technol. 187:44-52. doi 10.1016/j.anifeedsci.2013.09.009
Almeida, F. N., J. K. Htoo, J. Thomson, and H. H. Stein. 2014c. Effects of balancing crystalline amino acids in diets containing heat-damaged soybean meal or distillers dried grains with solubles fed to weanling pigs. Animal 8:1594-1602. doi 10.1017/S175173111400144X
Bothast, R. J., and M. A. Schlicher. 2005. Biotechnological processes for conversion of corn into ethanol. Appl. Microbiol. Biotechnol. 67:19-25. doi 10.1007/s00253-004-1819-8
Bujard, É., P. A. Finot, R. Madelaine, A. Lê van Kiet, R. Deutch, and A. Isely. 1978. Mesure de la disponibilité et du blocage de la lysine dans les laits industriels. Ann. Nutr. Alim. 32:291-
305.
Couch, J. R., and M. C. Thomas. 1976. A comparison of chemical methods for the determination of available lysine in various proteins. J. Agric. Food Chem. 24:943-946. doi
10.1021/jf60207a033
Cozannet, P., Y. Primot, C. Gady, J. P. Métayer, P. Callu, M. Lessire, F. Skiba, and J. Noblet.
2010. Ileal digestibility of amino acids in wheat distillers dried grains with solubles for pigs.
Anim. Feed Sci. Technol. 158:177-186. doi 10.1016/j.anifeedsci.2010.04.009
Chung, S. Y., S. H. Han, S. W. Lee, and C. Rhee. 2012. Effect of Maillard reaction products prepared from glucose–glycine model systems on starch digestibility. Starch - Stärke
64:657-664. doi https://doi.org/10.1002/star.201100176
Dworschák, E. 1980. Nonenzyme browning and its effect on protein nutrition. Crit. Rev. Food
Sci. Nutr. 13:1-40. doi 10.1080/10408398009527292
Eklund, M., N. Sauer, F. Schöne, U. Messerschmidt, P. Rosenfelder, J. K. Htoo, and R.
Mosenthin. 2015. Effect of processing of rapeseed under defined conditions in a pilot plant on chemical composition and standardized ileal amino acid digestibility in rapeseed meal for pigs. J. Anim. Sci. 93:2813-2825. doi 10.2527/jas.2014-8210
Finot, P., and E. Magnenat. 1981. Metabolic transit of early and advanced Maillard products.
Prog. Food Nutr. Sci. 5:193-207.
Fontaine, J., U. Zimmer, P. J. Moughan, and S. M. Rutherfurd. 2007. Effect of heat damage in an autoclave on the reactive lysine contents of soy products and corn distillers dried grains with solubles. Use of the results to check on lysine damage in common qualities of these ingredients. J. Agric. Food Chem. 55:10737-10743. doi 10.1021/jf071747c
Gerrard, J. A. 2002. New aspects of an ageing chemistry - recent developments concerning the
Maillard reaction. Aust. J. Chem. 55:299-310. doi 10.1071/CH02076
Goebel, K. P., and H. Stein. 2011. Ileal digestibility of amino acids in conventional and lowkunitz soybean products fed to weanling pigs. Asian-Australas J. Anim. Sci. 24. doi
10.5713/ajas.2011.90583
González-Vega, J. C., B. G. Kim, J. K. Htoo, A. Lemme, and H. H. Stein. 2011. Amino acid digestibility in heated soybean meal fed to growing pigs. J. Anim. Sci. 89:3617-3625. doi
10.2527/jas.2010-3465
Hirano, F., H. Kato, and M. Fujimaki. 1973. Racemization of amino acid residues in proteins during roasting. Agric. Biol. Chem. 37:191-192. doi 10.1080/00021369.1973.10860651
Hodge, J. E. 1953. Dehydrated foods, chemistry of browning reactions in model systems. J.
Agric. Food Chem. 1:928-943. doi 10.1021/jf60015a004
Kerr, B. J., T. A. Kellner, and G. C. Shurson. 2015. Characteristics of lipids and their feeding value in swine diets. J. Anim. Sci. Biotechnol. 6:30. doi:10.1186/s40104-015-0028-x
Kim, B. G., D. Y. Kil, Y. Zhang, and H. H. Stein. 2012. Concentrations of analyzed or reactive lysine, but not crude protein, may predict the concentration of digestible lysine in distillers dried grains with solubles fed to pigs. J. Anim. Sci. 90:3798-3808. doi 10.2527/jas.2011-
4692
Kimmel, J. R. 1967. Guanidination of proteins. Pages 584-589 in Methods in Enzymology
Academic Press.
Langner, E., and W. Rzeski. 2014. Biological properties of melanoidins: A review. Int. J. Food
Prop. 17:344-353. doi 10.1080/10942912.2011.631253
Liener, I. E. 1994. Implications of antinutritional components in soybean foods. Crit. Rev. Food
Sci. Nutr. 34:31-67. doi 10.1080/10408399409527649
Meade, S. J., E. A. Reid, and J. A. Gerrard. 2005. The impact of processing on the nutritional quality of food proteins. J. AOAC. Int. 88:904-922.
Mehta, B. M., and H. C. Deeth. 2016. Blocked lysine in dairy products: Formation, occurrence, analysis, and nutritional implications. Compr. Rev. Food Sci. Food Saf. 15:206-218. doi
10.1111/1541-4337.12178
Moughan, P. J. 2003. Amino acid availability: Aspects of chemical analysis and bioassay methodology. Nutr. Res. Rev. 16:127-141. doi 10.1079/NRR200365
Nursten, H. 2005. The Maillard Reaction. Chemistry, Biochemistry, and Implications. Royal
Society of Chemistry, Cambridge, UK.
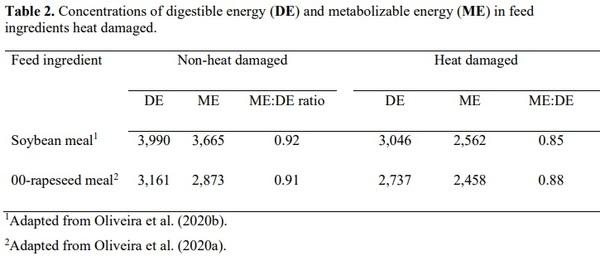
Oliveira, M. S. F., M. K. Wiltafsky-Martin, and H. H. Stein. 2020a. Excessive heating of 00- rapeseed meal reduces not only amino acid digestibility but also metabolizable energy when fed to growing pigs. J. Anim. Sci. 98. doi 10.1093/jas/skaa219
Oliveira, M. S. F., M. K. Wiltafsky, S. A. Lee, W. B. Kwon, and H. H. Stein. 2020b.
Concentrations of digestible and metabolizable energy and amino acid digestibility by growing pigs may be reduced by autoclaving soybean meal. Anim. Feed Sci. Technol.
269:114621. doi https://doi.org/10.1016/j.anifeedsci.2020.114621
Pahm, A. A., C. Pedersen, and H. H. Stein. 2008. Application of the reactive lysine procedure to estimate lysine digestibility in distillers dried grains with solubles fed to growing pigs. J.
Agric. Food Chem. 56:9441-9446. doi 10.1021/jf801618g
Pahm, A. A., C. Pedersen, and H. H. Stein. 2009. Standardized ileal digestibility of reactive lysine in distillers dried grains with solubles fed to growing pigs. J. Agric. Food Chem.
57:535-539. doi 10.1021/jf802047d
Patience, J. F., M. C. Rossoni-Serão, and N. A. Gutiérrez. 2015. A review of feed efficiency in swine: Biology and application. J. Anim. Sci. Biotechnol. 6. doi 10.1186/s40104-015-0031-
2
Ramírez-Jiménez, A., B. García-Villanova, and E. Guerra-Hernández. 2001. Effect of toasting time on the browning of sliced bread. J. Sci. Food Agric. 81:513-518. doi 10.1002/jsfa.840
Rehman, Z., and W. H. Shah. 2005. Thermal heat processing effects on antinutrients, protein and starch digestibility of food legumes. Food Chem. 91:327-331. doi
10.1016/j.foodchem.2004.06.019
Rutherfurd, S. M., and G. S. Gilani. 2009. Amino acid analysis. Curr. Protoc. Protein Sci.
58:11.19.11-11.19.37. doi 10.1002/0471140864.ps1109s58
Stein, H. H., S. P. Connot, and C. Pedersen. 2009. Energy and nutrient digestibility in four sources of distillers dried grains with solubles produced from corn grown within a narrow geographical area and fed to growing pigs. Asian-Australas J. Anim. Sci. 22:1016-1025. doi
10.5713/ajas.2009.80484
Zhang, Q., J. M. Ames, R. D. Smith, J. W. Baynes, and T. O. Metz. 2009. A perspective on the
Maillard reaction and the analysis of protein glycation by mass spectrometry: Probing the pathogenesis of chronic disease. J. Proteome Res. 8:754-769. doi 10.1021/pr800858h








