Ensiling of maize and Ensiling of tropical grass
Ensiling maize, tropical grass and big bale oat silages with inoculants in South Africa
Published: March 27, 2007
By: ROBIN MEESKE - South Africa Dept. of Economic Affairs (Courtesy of Alltech Inc.)
In South Africa summer rainfall occurs in the northern parts and winter rainfall in the southern parts. In some areas of the Southern Cape, rainfall varies from 750 to 1200 mm annually and rain occurs throughout the year. Even in this area farmers need to irrigate pastures as total rainfall and rain distribution vary considerably from year to year. To achieve the continuous supply of high quality roughage that forms the basis of profitable dairy farming, crops or grasses must be conserved either as standing forage, harvested hay or silage for use during the dry season.
The quality of silage depends on the quality of the crop at ensiling, type of fermentation, rate of pH decrease, moisture content of the crop and the ability to reach and maintain anaerobic conditions. The rate of pH decrease is determined by the level of water soluble carbohydrates (WSC) and the epiphytic bacterial numbers present on the crop prior to ensiling (McDonald et al., 1991; Rooke, 1990). Lactic acid bacteria therefore play a very important role in successful ensiling of crops.
In South Africa no survey has been done to determine the numbers of epiphytic microflora present on crops prior to ensiling. It has been shown that climatic conditions do have an impact on the numbers of viable lactic acid bacteria. High numbers at ensiling correlate positively with environmental temperature and air humidity and negatively with solar radiation (Muck, 1989; Ruser, 1989). Ruser (1989) reported on a survey of 991 grass and 370 maize crop samples and found 46 to 59% of the lactic acid bacteria were homofermentative. By increasing the number of homofermentative lactic acid bacteria, more efficient fermentation may take place. The effect of adding lactic acid bacterial inoculants to silage crops under local conditions in South Africa may differ from that found in Europe, as solar radiation is much higher in South Africa. In this paper results of studies to determine the effect of lactic acid bacterial inoculants on maize, a tropical grass (Digitaria eriantha) and oat silage are presented.
Ensiling of maize
Maize silage is the major silage crop in South Africa and farmers are often recommended to apply inoculants to maize at ensiling. Honig and Daenicke (1993) have shown that even in easily ensilable maize, fermentation losses can be further reduced by the use of an inoculant. The average daily gain of bulls was increased; however the aerobic stability of the maize silage was reduced by one day in inoculated maize silage compared to untreated control silage. Addition of homofermentative lactic acid bacteria such as Lactobacillus plantarum can produce a silage that is unstable when exposed to air (Kung et al., 1991; Rust et al., 1989; Weinberg et al., 1993). Therefore aerobic stability of maize silage should always be determined when additives are evaluated.
Adding an inoculant containing Lactobacillus plantarum, L. bulgaricus, L. acidophilus plus amylase and cellulase enzymes to maize at ensiling did not result in a more rapid drop in pH or higher levels of lactic acid in maize silage (Meeske et al., 1998). In this study the pH was 3.5 for both silages.
The in vitro organic matter digestibility (IVOMD) and crude protein (CP) content was 70.0% and 9.0% for the control and 69.5% and 8.8% for the inoculated silages, respectively. No butyric acid was detected in either silage.
The control and the inoculated silages contained 3.1 and 3.4% acetic acid, respectively. The lactic acid content was 5.8 and 5.4% and the WSC content was 2.2 and 2.3% for control and inoculated silage, respectively.
Intake and growth of South African Mutton Merino lambs fed the inoculated and untreated maize silage diets were determined. Average daily gain of lambs fed a diet consisting of either 60% control or inoculated maize silage over a growth period of 60 days is given in Table 1. Although the laboratory study showed very little effect of adding a lactic acid bacterial inoculant to maize at ensiling, there was a definite tendency for lambs to consume more of the inoculated silage and grow at a faster rate. This resulted in a shortening of the fattening period by five days and a saving of 2.5 kg of concentrate and 5 kg of silage per lamb fed the inoculated silage.
Table 1. Performance of lambs (n=14) fed control and inoculated maize silage diets for a period of 60 days.*
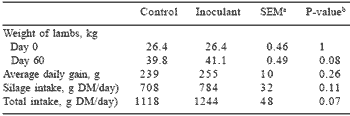
*Meeske et al. (1998)
a SEM = Standard error of means; bP-value = Level of significance.
In a second study maize silage either untreated or inoculated with Maize- All (Alltech Inc.) was fed to Jersey cows in an intake and milk production study. The inoculated and control maize silages made in bunker silos were both well preserved (Table 2). Dry matter (DM) content of 28 to 30% was optimal to ensure maximum dry matter intake (Phipps and Wilkinson, 1985).
The inoculated silage had a higher DM, nonstructural carbohydrate and CP content and a lower acetic acid content compared to that of the control silage. This may indicate a more efficient fermentation. The level of acetic acid of 27 g/kg DM and 20 g/kg DM found in the control and inoculated maize silages, respectively, was high compared to the 6 g/kg DM found by Scheafer et al. (1989) in bunker maize silage. Ashbell and Lisker (1988), however, reported acetic acid levels of 15 to 21 g/kg DM in maize silage made under farm conditions in a subtropical climate, while Spoelstra and Van Wikselaar (1992) found levels of 10 to 21 g acetic acid/kg DM.
Table 2. The chemical composition (g/kg DM) of control and inoculated bunker maize silages.
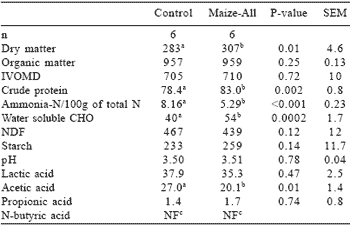
abMeans with different superscipts in the same row differ (P< 0.05).
cNot found
The starch content of inoculated maize silage did not differ from that of the control (Table 2). This indicated that the amylase did not break down starch as was found by Spoelstra and Van Wikselaar (1992) where the starch content of maize silage was reduced by up to 50% when enzymes with amylolytic activity were added to maize at ensiling. The WSC content of the inoculated maize silage was higher and acetic acid content lower than that of the control maize silage. This indicated that inoculation resulted in more efficient homofermentative fermentation. Neutral detergent fiber (NDF) content of the inoculated maize silage tended (P=0.12) to be lower than that of the control. This may be as a result of more hydrolysis of hemicellulose in the inoculated maize silage (McDonald et al., 1991).
Hemicellulose may be broken down during ensiling by hemicellulases present in the original herbage, bacterial hemicellulases and hydrolysis by organic acids produced during fermentation (McDonald et al., 1991).
Inoculation increased CP content of the silage (P
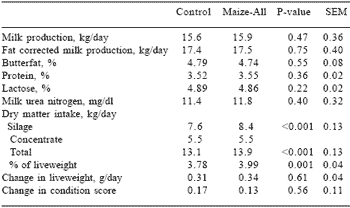
Milk production, fat corrected milk production and milk composition of cows did not differ significantly between the control and inoculated silage diets (Table 3). The intake of inoculated silage was significantly (P
Table 3. Intake, milk production and milk composition of Jersey cows (n=11) fed control or inoculated maize silage diets.
The control and inoculated bunker maize silages were stable for 7 and 39 hrs, 28 and 28 hrs, 8 and 19 hrs and 4 and 7 hrs during aerobic exposure tests 1, 2, 3 and 4, respectively. Aerobic deterioration of maize silage is initiated by yeasts or acetic acid bacteria (Driehuis and Van Wikselaar, 1996). The inoculated maize silage in our study was more stable than the control maize silage. This is in agreement with the study of Woolford (1975) and differs from the work of Moon et al. (1980) and Rust et al. (1989), who found that inoculated maize silage was less stable than untreated maize silage when exposed to air. Spoelstra and Van Wikselaar (1992) showed that the starch in maize silage is degraded relatively easy by amylase. The liberated sugars are then fermented to ethanol by yeasts, resulting in higher yeast counts and lowered aerobic stability of enzyme treated maize silage.
Scheafer et al. (1989) and Sanderson (1993) found that resistance to aerobic deterioration was unaffected by inoculation of maize silage with a lactic acid bacterial inoculant.
The number of yeast present on the crop at ensiling as well as the time from harvest until anaerobic conditions prevail has a major impact on the aerobic stability of maize silage. Our results show that a large variation in aerobic stability of maize silage occurred and that aerobic stability deteriorated as the feeding period proceeded. In a laboratory study both inoculated and untreated maize silages were stable when exposed to air for more than ten days. Conditions when making the laboratory maize silage were ideal.
Bunker maize silage on the other hand, which was made under less favourable conditions, was more susceptible to aerobic deterioration. Therefore, when evaluating the effect of additives on the aerobic stability of silage, laboratory studies should be followed by large-scale studies where silage is made on a commercial scale.
Ensiling of a tropical grass
The addition of inoculants to tropical grass (Digitaria eriantha) improved the fermentation dynamics during ensiling and composition of the silage shown in Table 4 (Meeske et al., 1999). The lactic acid content of inoculated silage was increased, the pH was lower and the acetic acid, butyric acid and ammonia-N as percentage of total N was lower than that of the control silage. Preservation of tropical grass silage made without the additive was poor. The improved preservation of D. eriantha resulted in increased IVOMD and silage intake by Merino rams (Table 5).
Table 4. The chemical composition (g/kgDM) of D. eriantha silage made in tower silos with or without a lactic acid bacterial inoculant versus that of hay*.
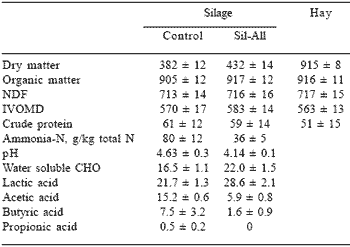
*The standard deviation (±) is given after the mean (n = 4).)
Table 5. In vivo organic matter digestibility and intake of D. eriantha silage and hay diets fed to mature Merino rams (n = 8 per treatment).
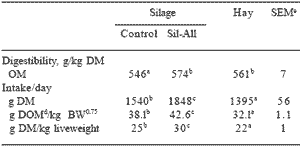
a,b,c Means with different letters in the same row differ significantly (P < 0.05).
d Digestible organic matter intake, e Standard error of means.
The addition of the inoculant did not have a detrimental effect on the aerobic stability of tropical grass silage. Although acetic acid content of inoculated tropical grass silage was lower than in the control, inoculated silage still contained more than 5.9 g acetic acid/kg DM. Weissbach (1996) evaluated 54 farm scale grass silages and found that when the acetic acid content of silages was lower than 3 g/kg DM, 64% of the silages were very unstable (
The addition of an efficient lactic acid bacterial inoculant to tropical grass at ensiling will ensure optimal use of the limited amount of water soluble carbohydrates available for fermentation. This will increase the rate of preservation and improve silage quality and intake of silage.
Ensiling big bale oat silage with or without inoculation
Oats (Avena sativa, cv Cederberg) were cut at the bloom stage, wilted to a 33 % DM, baled with a Krone KR130 baler and wrapped with a Kverneland wrapper (UN 7558) using Silawrap film (750 mm). The inoculant, Sil-All (Alltech Biotechnology Pty Ltd), contained Lactobacillus plantarum, Streptococcus faecium and Pediococcus acidilactici together with the enzymes cellulase, hemicellulase and amylase and was applied during the baling process with a pump and sprayer system at 10 g per tonne of fresh material on half of the bales. Bales were stored for nine months, transported to Outeniqua Experimental farm and fed to Jersey cows in an intake and milk production study. Twenty two multiparous cows were divided in 11 pairs with similar milk production (previous 4 weeks), days in milk, lactation number, liveweight and condition score. Within each pair, cows were randomly allocated to the control or inoculated silage treatments. The experimental period consisted of an adaptation period of 10 days followed by a measurement period of 21 days. Dry matter intake was determined on a daily basis. Cows were milked two times per day at 7:00 and 15:30. Three kilograms of a commercial dairy concentrate (230 g CP/kg and 12 MJ ME/ kg) were fed after each milking to all cows. The DM, OM, NDF, ADF, calcium (Ca), phosphorus (P), IVOMD and CP contents (g/kg DM) of the concentrate were 898 ±3, 918 ±6, 223 ±7, 80 ±3, 14 ±1, 8 ±0.1, 855 ±9 and 258 ±9, repectively. Silage was fed individually to cows twice daily at 8:00 and 16:30. Cows had access to silage from 8:00 to 12:00 and from 16:30 to 20:30. The composition of the oat silages is given in Table 6.
Table 6. The chemical composition (g/kg DM) of control and inoculated big round bale oat silages.
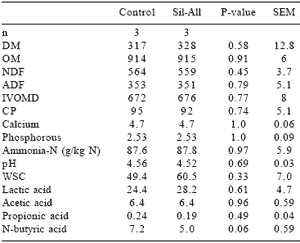
Inoculation resulted in lower levels of N-butyric acid compared to untreated silage. This indicates that the rate in pH decrease may have been faster in the inoculated silage. The inoculated big round bale oat silage did not have a significantly lower pH or higher lactic acid content compared to control silage as was found by Moshtaghi Nia and Wittenberg (1999) for inoculanttreated big bale whole crop barley ensiled at the early milk stage. The untreated and inoculated oat silages in our study were well preserved at a pH of 4.56 and 4.52 with DM contents of 317 and 328 g/kg silage, respectively. According to Weissbach (1996) the pH of silage should be 4.53 to ensure effective preservation when the DM content of a crop is 320 g/kg silage. Although the growth of most acid-tolerant clostridia is inhibited by a pH just below 5 (Jonsson, 1991), Jonsson et al. (1990) found that a DM of 350 g/kg in big bale grass silage was not high enough to give an acceptable reduction of clostridial activity. Jonsson et al. (1990) has shown that wilting and the use of additives increased the quality of big bale grass silage by a reduced pH, lower ammonia-N, butyric acid and clostridial spores.
The growth of Clostridium tyrobutyricum was not completely inhibited in silages treated with additives; but butyric acid production and sporulation were reduced. In our study, adding a lactic acid bacterial inoculant to oats ensiled in big bales resulted in lower levels of N-butyric acid compared to the control silage (Table 1), indicating a lower clostridial activity.
Inoculation had no effect on NDF and ADF content of big bale oat silage. This indicated that the enzyme complement did not cause a measurable amount of fibre breakdown. The NDF and ADF content of 564 and 353 g/ kg DM, respectively, for oat silage in this study compares well with the 525 g NDF/kg DM and 341 g ADF/ kg DM found by Moshtaghi Nia and Wittenberg (1999) for big bale whole crop barley silage. The WSC content of the control and inoculated silages was 49.4 and 60.5 g/kg DM, respectively, after nine months of ensiling, which indicates that WSC did not limit the rate of pH decrease and preservation in this study. When whole crop oats are harvested at the soft dough stage, the WSC content can be as low as 55 g/kg DM prior to ensiling (Jones et al., 1998), which may then limit the rate of preservation.
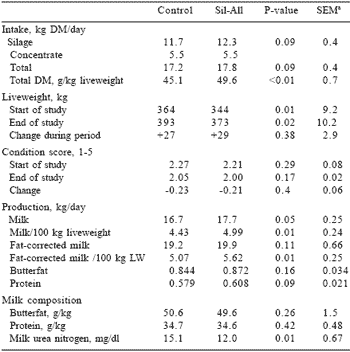
The inoculant treatment of big round bale oat silage increased milk production by 1 kg per cow per day and protein production tended (P=0.09) to be higher compared to the control (Table 7). Intake of inoculated silage also tended to be higher. No treatment differences were found in the butterfat and the protein content of milk. Chopping the big bale silage in a mixer wagon before feeding may have contributed to the high total DM intakes of 4.5 and 4.9% of liveweight for the control and inoculated silage diets, respectively. Liveweight change was unaffected.
Table 7. Feed intake, live weight change, condition score, milk production and milk composition of Jersey cows fed diets based on control or inoculated silages.
aSEM = standard error of mean
Inoculation of the silage significantly lowered milk urea nitrogen content compared to the control silage, suggesting a more efficient CP utilization. The lower N-butyric acid content found in the inoculated silage suggested a lower clostridial activity; and therefore less proteolysis could be expected. Ammonia-N (% of total N) content was, however, similar in control and treated silage.
Both the control and inoculated silages were stable when exposed to air and no heating of silage occurred during feeding. This can be explained by the acetic acid and N-butyric acid found in the silages, which would inhibit the growth of yeasts and moulds (Weissbach, 1996).
References
The quality of silage depends on the quality of the crop at ensiling, type of fermentation, rate of pH decrease, moisture content of the crop and the ability to reach and maintain anaerobic conditions. The rate of pH decrease is determined by the level of water soluble carbohydrates (WSC) and the epiphytic bacterial numbers present on the crop prior to ensiling (McDonald et al., 1991; Rooke, 1990). Lactic acid bacteria therefore play a very important role in successful ensiling of crops.
In South Africa no survey has been done to determine the numbers of epiphytic microflora present on crops prior to ensiling. It has been shown that climatic conditions do have an impact on the numbers of viable lactic acid bacteria. High numbers at ensiling correlate positively with environmental temperature and air humidity and negatively with solar radiation (Muck, 1989; Ruser, 1989). Ruser (1989) reported on a survey of 991 grass and 370 maize crop samples and found 46 to 59% of the lactic acid bacteria were homofermentative. By increasing the number of homofermentative lactic acid bacteria, more efficient fermentation may take place. The effect of adding lactic acid bacterial inoculants to silage crops under local conditions in South Africa may differ from that found in Europe, as solar radiation is much higher in South Africa. In this paper results of studies to determine the effect of lactic acid bacterial inoculants on maize, a tropical grass (Digitaria eriantha) and oat silage are presented.
Ensiling of maize
Maize silage is the major silage crop in South Africa and farmers are often recommended to apply inoculants to maize at ensiling. Honig and Daenicke (1993) have shown that even in easily ensilable maize, fermentation losses can be further reduced by the use of an inoculant. The average daily gain of bulls was increased; however the aerobic stability of the maize silage was reduced by one day in inoculated maize silage compared to untreated control silage. Addition of homofermentative lactic acid bacteria such as Lactobacillus plantarum can produce a silage that is unstable when exposed to air (Kung et al., 1991; Rust et al., 1989; Weinberg et al., 1993). Therefore aerobic stability of maize silage should always be determined when additives are evaluated.
Adding an inoculant containing Lactobacillus plantarum, L. bulgaricus, L. acidophilus plus amylase and cellulase enzymes to maize at ensiling did not result in a more rapid drop in pH or higher levels of lactic acid in maize silage (Meeske et al., 1998). In this study the pH was 3.5 for both silages.
The in vitro organic matter digestibility (IVOMD) and crude protein (CP) content was 70.0% and 9.0% for the control and 69.5% and 8.8% for the inoculated silages, respectively. No butyric acid was detected in either silage.
The control and the inoculated silages contained 3.1 and 3.4% acetic acid, respectively. The lactic acid content was 5.8 and 5.4% and the WSC content was 2.2 and 2.3% for control and inoculated silage, respectively.
Intake and growth of South African Mutton Merino lambs fed the inoculated and untreated maize silage diets were determined. Average daily gain of lambs fed a diet consisting of either 60% control or inoculated maize silage over a growth period of 60 days is given in Table 1. Although the laboratory study showed very little effect of adding a lactic acid bacterial inoculant to maize at ensiling, there was a definite tendency for lambs to consume more of the inoculated silage and grow at a faster rate. This resulted in a shortening of the fattening period by five days and a saving of 2.5 kg of concentrate and 5 kg of silage per lamb fed the inoculated silage.
Table 1. Performance of lambs (n=14) fed control and inoculated maize silage diets for a period of 60 days.*

*Meeske et al. (1998)
a SEM = Standard error of means; bP-value = Level of significance.
In a second study maize silage either untreated or inoculated with Maize- All (Alltech Inc.) was fed to Jersey cows in an intake and milk production study. The inoculated and control maize silages made in bunker silos were both well preserved (Table 2). Dry matter (DM) content of 28 to 30% was optimal to ensure maximum dry matter intake (Phipps and Wilkinson, 1985).
The inoculated silage had a higher DM, nonstructural carbohydrate and CP content and a lower acetic acid content compared to that of the control silage. This may indicate a more efficient fermentation. The level of acetic acid of 27 g/kg DM and 20 g/kg DM found in the control and inoculated maize silages, respectively, was high compared to the 6 g/kg DM found by Scheafer et al. (1989) in bunker maize silage. Ashbell and Lisker (1988), however, reported acetic acid levels of 15 to 21 g/kg DM in maize silage made under farm conditions in a subtropical climate, while Spoelstra and Van Wikselaar (1992) found levels of 10 to 21 g acetic acid/kg DM.
Table 2. The chemical composition (g/kg DM) of control and inoculated bunker maize silages.

abMeans with different superscipts in the same row differ (P< 0.05).
cNot found
The starch content of inoculated maize silage did not differ from that of the control (Table 2). This indicated that the amylase did not break down starch as was found by Spoelstra and Van Wikselaar (1992) where the starch content of maize silage was reduced by up to 50% when enzymes with amylolytic activity were added to maize at ensiling. The WSC content of the inoculated maize silage was higher and acetic acid content lower than that of the control maize silage. This indicated that inoculation resulted in more efficient homofermentative fermentation. Neutral detergent fiber (NDF) content of the inoculated maize silage tended (P=0.12) to be lower than that of the control. This may be as a result of more hydrolysis of hemicellulose in the inoculated maize silage (McDonald et al., 1991).
Hemicellulose may be broken down during ensiling by hemicellulases present in the original herbage, bacterial hemicellulases and hydrolysis by organic acids produced during fermentation (McDonald et al., 1991).
Inoculation increased CP content of the silage (P
Milk production, fat corrected milk production and milk composition of cows did not differ significantly between the control and inoculated silage diets (Table 3). The intake of inoculated silage was significantly (P
Table 3. Intake, milk production and milk composition of Jersey cows (n=11) fed control or inoculated maize silage diets.

The control and inoculated bunker maize silages were stable for 7 and 39 hrs, 28 and 28 hrs, 8 and 19 hrs and 4 and 7 hrs during aerobic exposure tests 1, 2, 3 and 4, respectively. Aerobic deterioration of maize silage is initiated by yeasts or acetic acid bacteria (Driehuis and Van Wikselaar, 1996). The inoculated maize silage in our study was more stable than the control maize silage. This is in agreement with the study of Woolford (1975) and differs from the work of Moon et al. (1980) and Rust et al. (1989), who found that inoculated maize silage was less stable than untreated maize silage when exposed to air. Spoelstra and Van Wikselaar (1992) showed that the starch in maize silage is degraded relatively easy by amylase. The liberated sugars are then fermented to ethanol by yeasts, resulting in higher yeast counts and lowered aerobic stability of enzyme treated maize silage.
Scheafer et al. (1989) and Sanderson (1993) found that resistance to aerobic deterioration was unaffected by inoculation of maize silage with a lactic acid bacterial inoculant.
The number of yeast present on the crop at ensiling as well as the time from harvest until anaerobic conditions prevail has a major impact on the aerobic stability of maize silage. Our results show that a large variation in aerobic stability of maize silage occurred and that aerobic stability deteriorated as the feeding period proceeded. In a laboratory study both inoculated and untreated maize silages were stable when exposed to air for more than ten days. Conditions when making the laboratory maize silage were ideal.
Bunker maize silage on the other hand, which was made under less favourable conditions, was more susceptible to aerobic deterioration. Therefore, when evaluating the effect of additives on the aerobic stability of silage, laboratory studies should be followed by large-scale studies where silage is made on a commercial scale.
Ensiling of a tropical grass
The addition of inoculants to tropical grass (Digitaria eriantha) improved the fermentation dynamics during ensiling and composition of the silage shown in Table 4 (Meeske et al., 1999). The lactic acid content of inoculated silage was increased, the pH was lower and the acetic acid, butyric acid and ammonia-N as percentage of total N was lower than that of the control silage. Preservation of tropical grass silage made without the additive was poor. The improved preservation of D. eriantha resulted in increased IVOMD and silage intake by Merino rams (Table 5).
Table 4. The chemical composition (g/kgDM) of D. eriantha silage made in tower silos with or without a lactic acid bacterial inoculant versus that of hay*.

*The standard deviation (±) is given after the mean (n = 4).)
Table 5. In vivo organic matter digestibility and intake of D. eriantha silage and hay diets fed to mature Merino rams (n = 8 per treatment).

a,b,c Means with different letters in the same row differ significantly (P < 0.05).
d Digestible organic matter intake, e Standard error of means.
The addition of the inoculant did not have a detrimental effect on the aerobic stability of tropical grass silage. Although acetic acid content of inoculated tropical grass silage was lower than in the control, inoculated silage still contained more than 5.9 g acetic acid/kg DM. Weissbach (1996) evaluated 54 farm scale grass silages and found that when the acetic acid content of silages was lower than 3 g/kg DM, 64% of the silages were very unstable (
The addition of an efficient lactic acid bacterial inoculant to tropical grass at ensiling will ensure optimal use of the limited amount of water soluble carbohydrates available for fermentation. This will increase the rate of preservation and improve silage quality and intake of silage.
Ensiling big bale oat silage with or without inoculation
Oats (Avena sativa, cv Cederberg) were cut at the bloom stage, wilted to a 33 % DM, baled with a Krone KR130 baler and wrapped with a Kverneland wrapper (UN 7558) using Silawrap film (750 mm). The inoculant, Sil-All (Alltech Biotechnology Pty Ltd), contained Lactobacillus plantarum, Streptococcus faecium and Pediococcus acidilactici together with the enzymes cellulase, hemicellulase and amylase and was applied during the baling process with a pump and sprayer system at 10 g per tonne of fresh material on half of the bales. Bales were stored for nine months, transported to Outeniqua Experimental farm and fed to Jersey cows in an intake and milk production study. Twenty two multiparous cows were divided in 11 pairs with similar milk production (previous 4 weeks), days in milk, lactation number, liveweight and condition score. Within each pair, cows were randomly allocated to the control or inoculated silage treatments. The experimental period consisted of an adaptation period of 10 days followed by a measurement period of 21 days. Dry matter intake was determined on a daily basis. Cows were milked two times per day at 7:00 and 15:30. Three kilograms of a commercial dairy concentrate (230 g CP/kg and 12 MJ ME/ kg) were fed after each milking to all cows. The DM, OM, NDF, ADF, calcium (Ca), phosphorus (P), IVOMD and CP contents (g/kg DM) of the concentrate were 898 ±3, 918 ±6, 223 ±7, 80 ±3, 14 ±1, 8 ±0.1, 855 ±9 and 258 ±9, repectively. Silage was fed individually to cows twice daily at 8:00 and 16:30. Cows had access to silage from 8:00 to 12:00 and from 16:30 to 20:30. The composition of the oat silages is given in Table 6.
Table 6. The chemical composition (g/kg DM) of control and inoculated big round bale oat silages.

Inoculation resulted in lower levels of N-butyric acid compared to untreated silage. This indicates that the rate in pH decrease may have been faster in the inoculated silage. The inoculated big round bale oat silage did not have a significantly lower pH or higher lactic acid content compared to control silage as was found by Moshtaghi Nia and Wittenberg (1999) for inoculanttreated big bale whole crop barley ensiled at the early milk stage. The untreated and inoculated oat silages in our study were well preserved at a pH of 4.56 and 4.52 with DM contents of 317 and 328 g/kg silage, respectively. According to Weissbach (1996) the pH of silage should be 4.53 to ensure effective preservation when the DM content of a crop is 320 g/kg silage. Although the growth of most acid-tolerant clostridia is inhibited by a pH just below 5 (Jonsson, 1991), Jonsson et al. (1990) found that a DM of 350 g/kg in big bale grass silage was not high enough to give an acceptable reduction of clostridial activity. Jonsson et al. (1990) has shown that wilting and the use of additives increased the quality of big bale grass silage by a reduced pH, lower ammonia-N, butyric acid and clostridial spores.
The growth of Clostridium tyrobutyricum was not completely inhibited in silages treated with additives; but butyric acid production and sporulation were reduced. In our study, adding a lactic acid bacterial inoculant to oats ensiled in big bales resulted in lower levels of N-butyric acid compared to the control silage (Table 1), indicating a lower clostridial activity.
Inoculation had no effect on NDF and ADF content of big bale oat silage. This indicated that the enzyme complement did not cause a measurable amount of fibre breakdown. The NDF and ADF content of 564 and 353 g/ kg DM, respectively, for oat silage in this study compares well with the 525 g NDF/kg DM and 341 g ADF/ kg DM found by Moshtaghi Nia and Wittenberg (1999) for big bale whole crop barley silage. The WSC content of the control and inoculated silages was 49.4 and 60.5 g/kg DM, respectively, after nine months of ensiling, which indicates that WSC did not limit the rate of pH decrease and preservation in this study. When whole crop oats are harvested at the soft dough stage, the WSC content can be as low as 55 g/kg DM prior to ensiling (Jones et al., 1998), which may then limit the rate of preservation.
The inoculant treatment of big round bale oat silage increased milk production by 1 kg per cow per day and protein production tended (P=0.09) to be higher compared to the control (Table 7). Intake of inoculated silage also tended to be higher. No treatment differences were found in the butterfat and the protein content of milk. Chopping the big bale silage in a mixer wagon before feeding may have contributed to the high total DM intakes of 4.5 and 4.9% of liveweight for the control and inoculated silage diets, respectively. Liveweight change was unaffected.
Table 7. Feed intake, live weight change, condition score, milk production and milk composition of Jersey cows fed diets based on control or inoculated silages.

aSEM = standard error of mean
Inoculation of the silage significantly lowered milk urea nitrogen content compared to the control silage, suggesting a more efficient CP utilization. The lower N-butyric acid content found in the inoculated silage suggested a lower clostridial activity; and therefore less proteolysis could be expected. Ammonia-N (% of total N) content was, however, similar in control and treated silage.
Both the control and inoculated silages were stable when exposed to air and no heating of silage occurred during feeding. This can be explained by the acetic acid and N-butyric acid found in the silages, which would inhibit the growth of yeasts and moulds (Weissbach, 1996).
References
Ashbell, G. and N. Lisker. 1988. Aerobic deterioration in maize silage stored in a bunker silo under farm conditions in a subtropical climate. J. Sci. Food Agric. 45:307.
Cilliers, J.W., H.J. Cilliers and W.R.L. Nel. 1998. Maize silage, sorghum silage and forage sorghum silage in diets with different portions of concentrate for the finishing of weaner lambs. Anim. Sci. 66:189.
Driehuis, F. and P.G. Van Wikselaar. 1996. Effects of addition of formic, acetic or propionic acid to maize silage and low dry matter grass silage on the microbial flora and aerobic stability. Proceedings of the 11th International Silage Conference, University of Wales, Aberystwyth (D.I.H. Jones, R. Jones, R. Dewurst, R. Merry and P.M. Haigh, eds.), p. 256.
Honig, H. and R. Deanicke. 1993. The effect of inoculation with lactic acid bacteria on the utilization of maize silage by bulls. Proceedings of the 10th International Silage Conference held from 6 to 8 September, 1993, at Dublin City University (P.O’Kiely, M. O’Connel and J. Murphy, eds). Dublin City University, p. 129.
Jones, E.L., J.E. Jones, R. Fychan, P. Bowling and R. Jones. 1998. Whole crop barley and oats undersown with Italian ryegrass/red clover as big bale silage in an organic system. In: Alternative Forages for ruminants (G.P.F. Lane and J.M. Wilkinson, eds.), Chalcombe Publications, p. 73.
Jonsson, A. 1991. Growth of Clostridium tyrobutyricum during fermentation and aerobic deterioration of grass silage. J. Sci. Food Agric. 54:557.
Jonsson, A, H. Lindberg, S. Sundas, P. Lingvall and S. Lindgren. 1990. Effect of additives on the quality of big-bale silage. Anim. Feed Sci. Technol. 31:139.
Kung, L., Jr., R.S. Tung and K. Maciorowski. 1991. Effect of a microbial inoculant and/or glycopeptide antibiotic on fermentation and aerobic stability of wilted alfalfa silage. Anim. Feed Sci. Technol. 35:37.
McDonald, P., A.R. Henderson and S.J.E. Heron. 1991. The Biochemistry of Silage, second edition. Chalcombe Publications, Marlow, Bucks, UK, p. 23.
Meeske, R. and H.M. Basson. 1998. The effect of a lactic acid bacterial inoculant on maize silage. Anim. Feed Sci. Technol. 70:239.
Meeske, R., H.M. Basson and C.W. Cruywagen. 1999. The effect of a lactic acid bacterial inoculant with enzymes on the fermentation dynamics, intake and digestibility of Digitaria eriantha silage. Anim. Feed Sci. Technol. 81:237.
Meeske, R., H.M. Basson, J.P. Pienaar and C.W. Cruywagen. 2000. A comparison of the yield, nutritional value and predicted production potential of different maize hybrids for silage production. South Afr. J. Anim. Sci. 30(1):18.
Moon, N.J., L.O. Ely and E.M. Sudweeks. 1980. Aerobic deterioration of wheat, lucerne and maize silages prepared with Lactobacillus acidophilus and a Candida spp. J. Appl. Bacteriol. 49:75.
Moshtaghi Nia, S.A. and K.M. Wittenberg. 1999. Use of forage inoculants with or without enzymes to improve preservation and quality of whole crop barley forage ensiled as large bales. Can. J. Anim.Sci. 79:525.
Muck, R.E.1989. Initial bacterial numbers on lucerne prior to ensiling. Grass Forage Sci. 44:19.
Phipps and M. Wilkinson. 1985. Maize silage for dairy cows. In: Maize Silage. Chalcombe Publications, UK, pp. 29.
Rooke, J.A. 1990. The numbers of epiphytic bacteria on grass at ensilage on commercial farms. J. Sci. Food Agric. 51:525.
Ruser, B. 1989. Das vorkommen von lactobacterien auf futterpflanzen. Landbauforschung Völkenrode, 39:32.
Rust, S.R., H.S. Kim and G.L. Enders. 1989. Effects of a microbial inoculant on fermentation characteristics and nutritional value of corn silage. J. Prod. Agric. 2:235.
Sanderson, M.A. 1993. Aerobic stability and in vitro digestibility of microbially inoculated corn and sorghum silages. J. Anim. Sci. 71:505.
Schaefer, D.M., P.G. Brotz, S.C. Arp and D.K. Cook. 1989. Inoculation of corn silage and high-moisture corn with lactic acid bacteria and its effects on the subsequent fermentations and on feedlot performance of beef steers. Anim. Feed Sci. Technol. 25:23.
Spoelstra, S.F. and P.G. Van Wikselaar. 1992. The effects of ensiling whole crop maize with a multi-enzyme preparation on the chemical composition of the resulting silages. J. Sci. Food Agric. 60:223.
Weinberg, Z.G., G. Ashbell, Y. Hen and A. Azrieli. 1993. The effect of applying lactic acid bacteria on the aerobic stability of silages. J. Appl. Bacteriol. 75:512.
Weissbach, F. 1996. New developments in crop preservation. Proceedings of the 11th International Silage Conference held at the University of Wales, Aberystwyth, September 8-11, 1996 (D.I.H. Jones; R. Jones, R. Dewurst, R. Merry and P.M. Haigh, eds.), p. 11.
Woolford, M.K. 1975. Microbiological screening of the straight chain fatty acids (C1-C12) as potential silage additives. J. Sci. Food Agric. 26:219.
Author: ROBIN MEESKE
Department of Economic Affairs, Agriculture and Tourism, Western Cape, Outeniqua Experimental Farm, George, South Africa.
Department of Economic Affairs, Agriculture and Tourism, Western Cape, Outeniqua Experimental Farm, George, South Africa.
Related topics:
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.





.jpg&w=3840&q=75)