differences between phytase enzymes in practice
Latest published research in phytate-protein interactions highlights differences between phytase enzymes in practice
Published: September 13, 2012
By: Peter Plumstead (Danisco Animal Nutrition, UK) and Shukun Yu (Enzyme R&D, Genencor, Danisco A/S, Aarhus, Denmark)
A breakthrough in our understanding of phytase enzyme mode of action and the associated matrix values in feed formulation has been provided in a recent paper published by Tran et al. in the January 2011 edition of Analytical Biochemistry entitled 'A simple and fast kinetic assay for phytases using phytic acid–protein complex as substrate'.
While at first glance the relevance of the title to animal nutritionists may not be immediately obvious, this collaborative research by scientists from Lund University (Sweden) and Genencor studied interactions of phytate with dietary proteins as measured by the formation of insoluble complexes and the suitability of using the phytate-protein complex as a substrate for phytase assays. The authors demonstrated that, as phytate-protein complexes occurred in feed and the digestive tract of animals, this may be a more relevant substrate for the evaluation of phytases than sodium phytate, a laboratory chemical routinely used in traditional phytase assays. Of commercial interest was that the study included an independent evaluation of four different commercial phytases in their effectiveness to hydrolyze either synthetic sodium phytate, or naturally occurring protein-phytate complexes in feed at a pH of 3.0. Results shown in Table 1 firstly confirm that when sodium phytate (IP6-Na) is used as a substrate at low pH, the degree of Na-phytate hydrolysis is similar between the two E. coli phytases, but is significantly lower when fungal phytases (P. lycii and A. niger) are applied. These differences between E. coli and fungal phytase sources are not new and have been shown to be a function of the lower pH optimum of the E. coli phytases versus their fungal phytase counterparts (Wyss et al., 1999). However, of greater relevance to nutritionists are the large differences shown between commercial phytases in their ability to degrade protein-phytate complexes, being not only significantly different between E. coli and fungal phytases, but also between different E. coli phytase sources. As the main anti-nutritional effect of phytate is caused by the formation of insoluble protein-phytate complexes in the acid stomach this data provides the first strong evidence that the nutritional benefit from phytase enzymes can no longer be viewed as being constant within classes of phytase such as E. coli. Consequently, in practice, different matrix values should be applied in feed formulation to account for these differences.
Table 1: Activity of different commercial phytases at pH 3.0 and 37°C on soy protein or lysozyme protein complexes with phytate (IP6) as compared with sodium-phytate (IP6-Na) as substrate. All phytases were added at a dose of 0.1 FTU/ml (fFrom Tran et al., 2011).
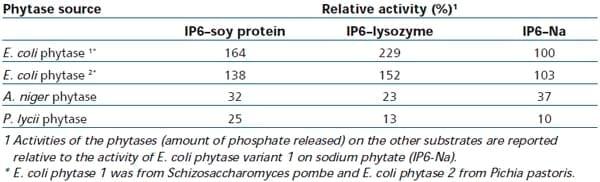
To fully understand the research results shown in Table 1 it is important to know that while sodium phytate is used as the substrate to determine phytase activity in-vitro, in nature and also in the acid part of the digestive tract of poultry (proventriculus and gizzard) and swine (stomach), phytic acid does not occur in its free acid or sodium salt form, but rather in association with proteins. The reason for the association of phytate and protein in the gut is that most proteins of plant origin, such as those derived from maize, soybean, sunflower and rapeseed (canola) meal, have their iso-electric points (pI values) in the acidic range (pH 4.5). Hence, when the pH in the digestive tract drops below the iso-electric point of the protein, the protein is now positively charged, which allows the negatively charged phytic acid molecule to bind to it, thereby altering the proteins iso-electric point and rendering the protein insoluble (Reddy et al., 1989; Konietzny and Greiner, 2003). This formation of insoluble protein-phytate complexes in the acid part of the digestive tract can have important nutritional consequences due to a decreased accessibility to pepsin proteases resulting in increased pepsin secretion, greater endogenous losses and inefficient protein digestion, as indicated by a reduced ileal amino acid digestibility from phytate. (Vaintraub and Bulmaga, 1991, Konietzny and Greiner, 2003, Kies et al., 2006, Cowieson et al., 2008).
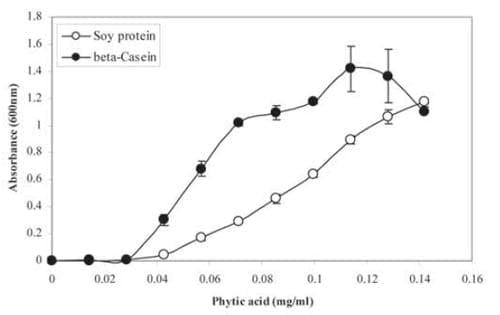
Of further importance are new data that have shown the formation of insoluble protein-phytate complexes to be proportional to the amount of phytate present. This is shown in Figure 1 where the presence of insoluble protein-phytate complexes from either soy protein or casein, measured by absorbance at 600 nm, increased almost linearly as the phytate concentration increased. Stated another way, the anti-nutritional effect of phytate in the acid stomach of the animal will be proportional to the concentration of undigested phytate (IP6) present in the acid stomach and is further supported by previous work by Cowieson et al. (2009), who demonstrated a step-wise increase in endogenous amino acid losses at the terminal ileum as dietary phytate levels increased. Converse to the negative effects of phytate on energy and amino acid digestibility, nutritional advantages of phytase enzymes will depend primarily on the ability of the source of phytase to rapidly hydrolyze phytate-protein complexes at a low pH (gizzard and provetriculus region), thereby reducing their anti-nutritional effects and resulting in net improvements in energy and protein utilization.
Feed costs too high?
This is just one of the challenges that Danisco can help you solve.
Phyzyme XP is a highly efficient phytase feed enzyme that improves the digestibility of phosphorus, calcium, energy and amino acids contained in many feed ingredients. Reduce your feed costs by minimising the need to add expensive inorganic phosphorus sources and reformulate the feed to take account of the improvements in energy and amino acid digestibility.
FEEDING
In conclusion, the past decade of research has shown anti-nutritional effects of phytate to extend beyond a simple reduction in phosphorus and calcium availability and include negative effects of phytate on protein-binding, endogenous nutrient losses, and energy and amino acid utilization. An understanding by nutritionists that the formation of insoluble protein-phytate complexes in the acid stomach is a primary mode of action whereby phytate exerts its anti-nutritional effects and conversely, whereby phytase enzymes contribute to improved energy and amino acid utilization is essential in order to make informed decisions on selecting appropriate phytase sources for in-feed application.
Figure 1: Formation of insoluble phytate-soy or phytate-casein complexes increases with increasing phytic acid concentration (from Yu et al., unpublished data).
The clear differences between the sources of phytase evaluated in the research by Tran and co-workers would suggest that the nutritional benefit of the different phytases cannot be the same, which makes a critical reassessment of the associated matrix values provided by the phytase suppliers imperative. Furthermore, as the effects of phytate on binding protein in the acid environment are also proportional to the phytate concentration and dose of phytase in feed, the matrix value applied to phytases in feed formulation can be summarised as being a function of these three factors and should be adjusted based on:
1. The source of phytase enzyme and its ability to hydrolyze phytate-protein complexes at low pH.
2. The phytate level of the diet, with reduced benefits in energy and amino acid utilization being derived from phytases at lower levels of dietary phytate.
3. Higher phytase doses provide a greater ratio of enzyme:substrate in the digesta, allowing for a more rapid degradation of the protein-phytate complex, further reducing its anti-nutritional effects and improving net dietary energy and amino acid utilization.
Key references
T. T. Tran, R. Hatti-Kaul, S. Dalsgaard, S. Yu. 2011. A simple and fast kinetic assay for phytases using phytic acid–protein complex as substrate. Anal. Biochem. 410: 177–184.
S. Yu, A. Cowieson, P. Plumstead, C. Gilbert, and S. Dalsgaard. Interactions of phytate and myo-inositol phosphate esters (IP1-5) including IP5 isomers with dietary proteins and iron and inhibition of pepsin.
Related topics:
Authors:
IFF - International Flavors & Fragrances
Chemuniqué
Recommend
Comment
Share
Farmer Joe Group
28 de noviembre de 2012
Does anyone know if Quantum Blue comes from the same Escherichia coli as Phyzyme XP? If so then the 2 products should perform similarly, and AB Vista's claim to superiority should be challenged !
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.











