Introduction
Silver has long been known to exhibit a strong toxic activity towards a wide range of microorganisms, for this reason silver based compounds have been extensively used in many bactericidal applications. Nano particles due to their large surface area are highly reactive and Ag Nano particles have been tested in various fields of biological sciences, Viz drug delivery, wound treatment, binding with HIV gp120 protein , in water treatment and as antibacterial compound against both Gram-positive and Gram-Negative bacteria. The exact anti-microbial mechanism of silver is not known; However, it has been determined that the free silver ion is the active agent, combining with the thiol (SH) groups, which leads to the protein inactivation. There is evidence that antibacterial potency of silver nano particle is directly proportional to the concentration of silver ions in the solution. In the present study we have reported about the influence of silver nano particles on antibacterial activity.
Materials and Methods
Bio-synthesis of silver nano particles
Silver nano particle synthesis was carried out by taking 500 mg of dry seaweed powder in 250 ml Erlenmeyer flask with 10-3 M aqueous (AgNO3-) solution and incubated at room temperature. The pH was checked during the course of reaction and it was found to be 5.09. The bio- synthesis of silver nanoparticles was characterized by UV Vis nanophotometer; size and morphology by employing SEM, structure from X-ray diffraction (XRD) technique and Fourier transform infrared (FT-IR) spectroscopy.
Bacterial Susceptibility to nano silver
The bacterial cultures were obtained from IMTECH, Chandigarh, India. Staphylococcus aureus, Salmonella typhi, Escherichia coli, Klebsiella pneumoniae and Proteus vulgaris are the strains used to study the efficacy of silver nano particles. Disc diffusion assay was performed in LB agar plates where 20 ml LB agar was poured in well rinsed, autoclaved petri plates, 1.0 ml of active bacterial culture was homogeneously spread in the agar plates and 30 µg/ml of silver nano particle was placed in the sterile disc which was placed on the LB agar along with the standard commercially available antibiotics of similar concentration. The plates were incubated at 37°C for 24 hrs.
Minimum inhibitory concentration
The bactericidal activity of Ag Nano particles was determined by minimal inhibitory concentration. Bacterial cells were grown in LB medium and 500 µl of 24 h - old bacterial culture (0.1 OD) was spreaded over the LB agar plates, supplemented with 5, 15, 30, 45 and 60 µg/ml of bared Ag nanoparticles. The plates were incubated for 24 h. Antimicrobial test compound below the MIC cannot inhibit microbial growth. The lowest concentration that inhibited the complete activity of microorganisms was recorded as minimum bactericidal concentration.
Results and Discussion
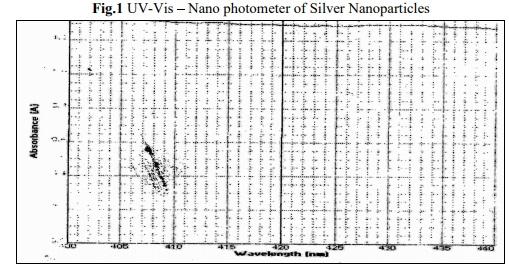
Silver nano particles are formed by the reduction of Ag+ during exposure to the extract at 600°C. Colour change from pale yellow to brown colour indicates the formation of silver nano particles in the solution and this may be due to the excitation of surface plasmon vibrations in the silver metal nano particles. Fig.1 shows the UV Nano photometer from the biosynthesized silver nano particles obtained from the extract of the marine red alga H.poryphyroides. It is observed that the silver surface plasmon resonance band occurs at 420 nm. The frequency and width of surface plasmon absorption depends upon the size and shape of the metal nanoparticles as well as on the dielectric constant of the metal itself and the surrounding metal. It is generally recognized that UV Nano photometer could be used to examine absorption peak of controlled nano particles in aqueous suspensions.
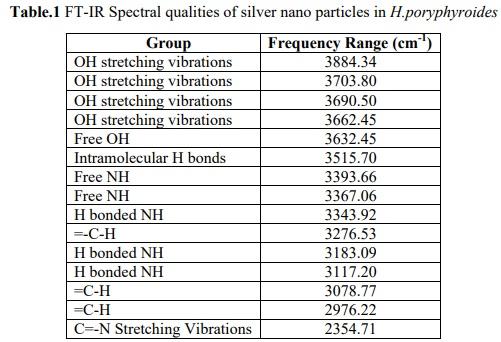
The FT-IR spectrum analysis of silver nano particles manifests absorption peaks. The possible potential biomolecules responsible for the reduction of silver ions to silver nanoparticle were identified using FT IR analysis. Figure 2 shows the FT IR spectrum of algal assisted silver nano particles. The spectral bands were interpreted for identification of functional moieties of organic compounds adhering to the silver nano particles. The band at 3662.45 to 3884.34 cm-1 represents O H stretching groups of amides plane bending respectively. The band at 3632.45 cm-1 corresponds to a free alcohol group, and the band at 3515.70 cm-1 corresponding to intramolecular hydrogen bonds. The band at 3367.06 to 3393.66 cm-1 free amine. The band at 3117.20 to 3343.92 cm-1 assigned to be H bonded NH. The band at 2976.22 to 3276.53 cm-1 assigned to be =- C-H. The band at 2354.71 cm-1 assigned to be C=-N stretching vibrations. The groups of polysaccharides which are found in the H.poryphyroides have their interaction in the synthesis process of silver nano particle. The biological molecules such as secondary metabolites could possibly play a major role in the synthesis and stabilization of the metal nano particles was proved by FT-IR analysis. The result revealed that the capping ligand of the Ag- NPs may be an aromatic compound of alkanes or amines.
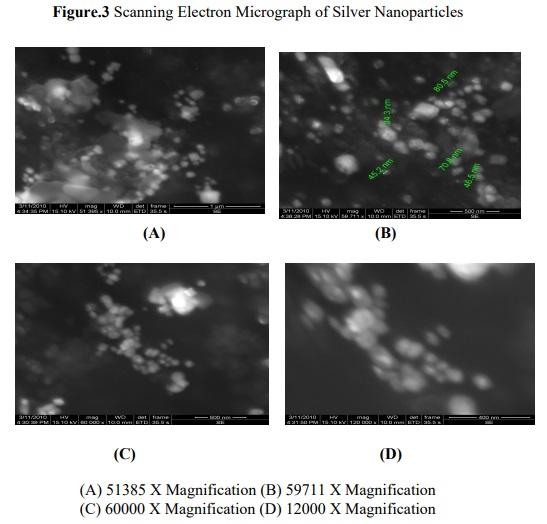
The SEM analysis of silver nano particles, besides being present in colloidal form in solution, was also micro precipitated on the surface of the biomass particles of H.poryphyroides. The Figure 3 shows the magnified view of algal assisted silver nano particles with the spherical shape and average size of the nano particle. The more stable spherical shape and isotropic nanoparticles was formed by the action of a large number of biomolecules ranged in the solution. The silver nano particles seems to be coated with the cell wall polysaccharide on the micrograph represented metallic silver which is reflected due to the diffraction of the electron beam from the metallic surface. It is known that the shape of the metal nano particles considerably changes their optical and electronic properties.
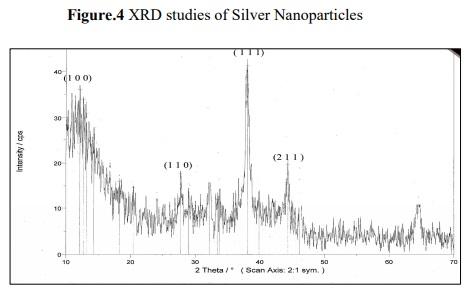
Figure 4 shows XRD patterns obtained from biosynthesized silver nano particles which shows characteristic peaks at (2 =22°), marked with {1 1 1}. Bragg reflections corresponding to {1 0 0}, {1 1 0}, {1 1 1} and {2 1 1} sets of lattice planes are observed in powder XRD pattern, which may be indexed based on the FCC structure of silver. The XRD pattern thus clearly shows that the silver nano particles are crystalline in nature. The value of pure silver lattice constant has been estimated to be = 4.081, a value that is consistent with =4.0862 A 0 reported by the JCPDS file no 4.0783. This estimation confirmed the hypothesis of particle mono crystallinity. The sharpening of the peaks clearly indicates that the particles are in nanoregime.
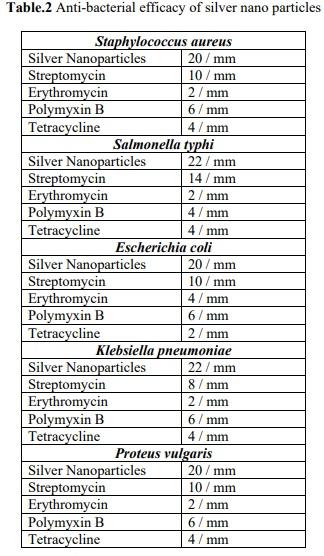
Silver nano particles synthesized from marine alga Halymenia poryphyroides were tested against bacterial pathogens Staphylococcus aureus, Salmonella typhi, Escherichia coli, Klebsiella pneumoniae and Proteus vulgaris and their efficacy were determined. The bacterial pathogens were susceptible to silver nano particles (30 µg/ml) in the range 20-22 mm in diameter as show in figure 5 compared to standard antibiotics Streptomycin, Erythromycin, polymyxin B and tetracycline. Minimum Inhibitory concentration was observed at a concentration of 5 µg/ml for all the organisms The efficacy of silver nano particles may be attributed by the phosphorylation of various proteins in the bacterial pathogen and is found to influence the bacterial signal transduction. The gram negative bacteria have a layer of

lipopolysaccharide at the exterior, followed underneath by a thin (about 7-8 nm) layer of peptidoglycan. They lack strength and rigidity although they are composed of covalently linked lipids and polysaccharides. Negative charges on the lipopolysaccharides are attracted towards weak positive charges available on silver nano particles. Gram positive cell wall is composed of a thick layer of peptidoglycan (20-80 nm) consisting of linear polysaccharide chains cross linked by short peptides to form three dimensional rigid structure. The rigidity and extended cross linking endow the cell walls with fewer anchoring sites and the efficacy varies depending upon the concentration of silver nano particles. Thus the silver nano particles showed a greater efficacy for bacterial pathogens as compared to commercially available antibiotics of the same concentration. This efficacy of silver nano particles may also be attributed to its use over the commercially available antibiotics which are less futile due to their overuse and becoming drug resistant. Therefore silver nano particles play an important role in antimicrobial activity. The above result concluded that the algal mediated AgNp s shows a wide range of biological activity against microorganisms which can be applied in the medical field in future.
This article was originally published in International Journal of Current Microbiology and Applied Sciences (2014) 3(4).















