Introduction
The practical and scientific knowledge obtained on the use of the Lactoperoxidase system (LPs) confirms its innocuity to human health (1), which allowed the lifting of the restriction that the system could not be used for milk products intended for international dairy market, in the Thirty-Second Session of the Commission Codex Alimentarius (2). The simple name of LPs generates confusion due to the association to hydrogen peroxide or oxygenated water, adding without scientifically sustained criteria on the microbiological, toxicological and technological hazards. Contrarily, there is a clear tendency about the use of the system for preserving meats, fruits and vegetables, substituting active chloride or other hazardous substances (3,4,5).
The most discussed aspects are:
- Toxic potential of sodium thiocyanate salt used on the exogenous activation of the system due to the possible interference on the iodine metabolism (6, 7).
- Possible risks of pathogen microorganisms exacerbation, once the systems is inactivated (1, 8, 9).
- Possible increase of the microorganisms resistance to the method (9).
- Loss of interest of dairy producers on hygiene practices (1).
A review on the scientific information available done by a Technical Committee from FAO/WHO (1), recommends that its use, in correspondence to the guidelines (10), is a way to stimulate the dairy development in areas in which the adequate infrastructure to apply refrigeration system does not exist. This work integrates several studies carried out in the last 20 years under Cuban and other tropical countries conditions and establishes a current and perspective approach to the subject.
1. Methodologic aspects
The analysis of thiocyanate ion (SCN-) content in cow bulk and individual raw milk was done in 1995 samples (895 and 1100 samples respectively), representing a total volume of 4 millions liters, using the method described in the guidelines of the Codex Alimentarius CAC/GC 13, (10). The study on bulk milk included herds from Cuba, Mexico and Venezuela.
The effect of activation upon the contaminant flora of raw milk included cows, goats, buffaloes and sheep. Twenty three assays were carried out, including the determination of different groups of microorganisms: aerobic viable mesophiles, coliforms, psychrotrophics, thermoresistants and proteolytics, with emphasis in the first two ones, at different activation times: 0, 2, 4, 8 and 12 hours at fluctuating room temperature, between 22-36 ºC, according to the guidelines (10). The description of the microbiological methods used corresponds with the international norms (11). For the exacerbation studies, raw milk experimentally contaminated with Salmonella typhimurium ATCC 14028, Staphylococcus aureus ATCC 25923, Escherichia coli enterohemorrágica O157:H7, Listeria monocytogenes ATCC 43256 and Bacillus cereus sp was used during the times previously pointed out. For the exacerbation criterion, it was considered that the amounts of pathogen microorganisms maintain the same counting (no significant differences), or when a reduction in time occurred, comparing the control milk with the LPs activated sample. An LPs activator product was used (11), containing the quantities of sodium thiocyanate and percarbonate salts established in the guidelines (Codex Alimentarius, 1991). Activation was done on 2 L milk aliquots (laboratory assays) milk jars of 40-50 L (dairy farm assays) and in 500-5000 L tanks (collecting assays). In all cases, homogeneous mixtures were used to obtain the control and treated samples.
The measure of the activation effect upon the milk components and products includes protein, fat and lactose determination by infrared method in hot raw milk and the final contamination in pasteurized milk. Assays on the final quality of yogurt and maturated cheeses were also included. In all cases, the evolution of the quality indicators inactivated and not activated milk was studied.
To evaluate the use of the LPs activation within an Integral Program for Milk Quality Improvement, the activation was carried out in 40 L jars or during the collecting process of 500-5000 L, from 700 herds, with a total of 36 000 cows, during seven years.
2. Thiocyanate Content in Raw Milk
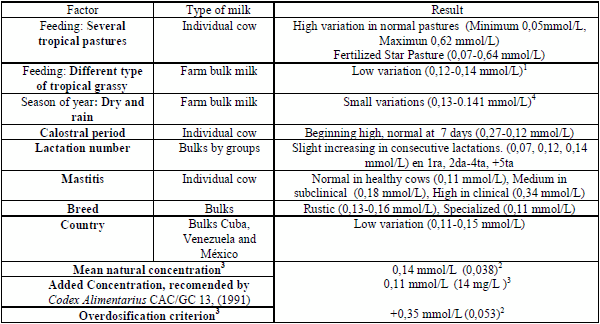
The variation of thiocyanate ion concentrations in milk from individual cows was very wide, with values from 0,05 mmol.L-1 to 0,62 mmol.L-1, but concentration in bulk milk was much lesser variable, with values maintained in a close range between 0,11-0,18 mmol.L-1, with an average of 0,14 mmol.L-1, repeated in the major part of observations (Table 1). Milk from cows consuming star pasture (Cynodon nlenfluensis) fertilized with nitrogen (experimental conditions), had the highest values, the concentrations in bulk milk of cows fed in dairy farms using different types of pasture, including star pasture, were lesser; and the average concentrations observed in the different countries were equally similar. The highest concentrations were found in cows with subclinical and clinical mastitis and in animals with more than five lactations.
A tendency to show higher concentrations was also observed in animals from rustic breedings such as Zebu and crossbreeding with Holstein and Brown Swiss. From these results, the general average concentration was established in 0,14 mmol.L-1 and the concentration needed for activation under the American tropic conditions in 0,11 mmol/L. To account its values, the concentration over 0,35 mmol.L-1, can be considered as thiocyanate overdose.
The results significantly contribute to a higher security and innocuity in the use of the LPs, since a lesser thiocyanate concentration than that indicated by the Codex Alimentarius Guidelines is used, due to the activation range is much lesser than the maximal natural concentration found in the milk of an individual animal. The obtainment of a limit value to consider an inadequate use of the LPs is also a criteria allowing its control of use.
Table 1. Factors associated to thiocyanate variation in cow raw milk1./ Factores asociados a la variación de tiocianato en leche cruda1
 1
1 Represents the values the ion SCN concentration in all the cases
2 Standard error (SE)
3This value represents the exogenous addition of 14 mg / L of sodium thiocyanate salt
4 Differences were not observed among times of the year
3. Activation of the Lactoperoxidase System
The essentially bacteriostatic nature of the LPS, avoids the quick multiplication of the milk contaminant saprophytic flora, lowering the deterioration of the initial quality and the losses due to acidification (1, 12, 13 ), demonstrated in all mammalian species of economic importance.
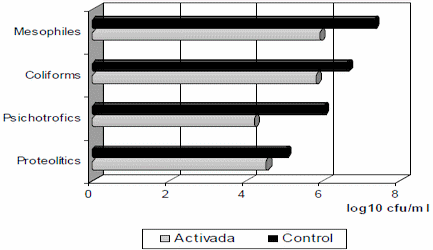
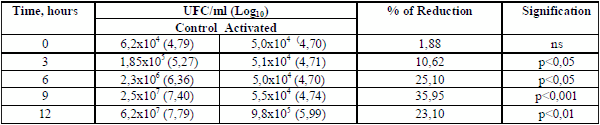
Changes in the mean values of cfu/mL in log10, the main groups of milk contaminant bacteria, measured at 4 hours post activation (Fig. 1), indicate a decrease of the total amount in the order of one log or higher, independently of the microorganism group, the initial contamination and milk temperature. The dynamics of the bactericidal effect in coliforms group (Table 2), shows that such effect is minimal in the first two hours, increases until 8-9 hours and decreases from the 12 hours, occurring concomitantly with the bacteriostatic activity, which is the main action of the system (14, 15,16, ).
1Means of 27 laboratory tests done during the years 1985-2003. *p<0,05), **p<0,01 between group in all cases.
Figure 1. Effect of the LP system activation on different microorganisms groups (log10 ufc/ ml) at 4 hours post activation1./ Efecto de la activación del sLP sobre diferentes grupos de microorganismos (log10 ufc/ml) a las 4 horas de post activación1.
Table 2. Dynamic of the LP system activation on the coliform bacteria counting1./ Dinámica de la activación del sLP sobre el conteo de bacterias coliformes1
 1
1Milk bulks of 5 producers with manual milking, maintained at room temperature between 27.6-34.9
0C. Three replicas by origin.
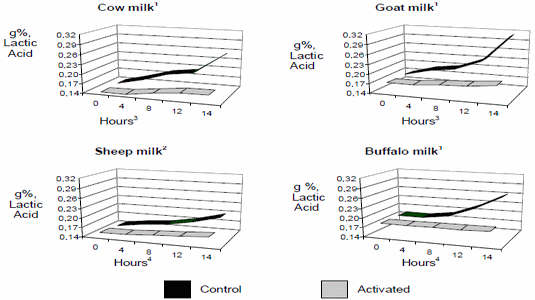
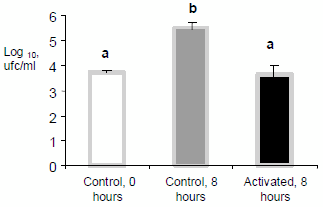
This effect has its expression in decreasing the lactic acid production capacity by lactofermentant bacteria, being the most visible practical base of the activation in hot milk in the tropic, since it avoids the acidification and curdled, either in cow, buffalo, goat and sheep (Fig. 2). The use of LPs in hot milk with excellent initial quality (less than 10 000 cfu/mL), indicates that such quality can be maintained stable for at least 8 hours at room temperature between 28-34oC, (Fig. 3), which supposes that the method does not have to be only directed to the conservation of raw milk with high contamination.
The LPs activation method is used in Cuba during the last 15 years in more than 1 200 millions of milk liters. Include cow, buffalo and goat milk in different production and collection conditions (Ponce, 2007). Also include experiences of use in 23 Latin American and Caribbean countries (1, 11).
1Mean values of 23 laboratory assays and field observations in different countries during the years 1988-2003.
2Milk of sheep, data of studies only from Albania. Fluctuating room ambient temperature between 22-360C.
3,4Significative differences (*p<0,05) starting from 4 hours in cow and goat and from 6 hours in buffalo and sheep.
Figure 2. Effect of the LP system activation on the titulable acidity in cow, goat, sheep and buffalo milk3./ Efecto de la activación del sLp sobre la acidez titulable en leche de vaca, cabras, ovejas y búfalas.
1Three replicas in alternate days. Different letters, p<0,001. Temperature between 27.7-33.10C.Volume of 517, 490 and 508 liters for control 0, control 8 and activated 8 hours, respectively
Figure 3. Effect of the LP system activation on the mesophile bacteria content in raw milk of excellent initial quality1./ Efecto de la activación del sLP sobre el contenido de bacterias aerobias mesófilas en leche cruda de excelente calidad inicial.
4. Exacerbation of the Foodborne Microorganisms
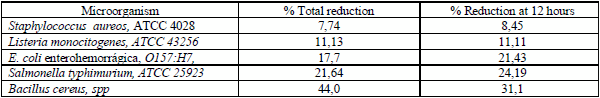
The results obtained from the experimental contamination of raw milk with strains pathogen microorganisms (Table 3), show that no significant differences were observed in any case between the activated and control milk, although the absolute concentration values were always lower in treated milk, at least in one reduction log. More that one reduction log was obtained at 8 hours with Listeria monocytogenes and at 8 and 12 hours with Escherichia coli.The reduction percent measured at 12 hours post activation was 8,45% in the case of Staphylococcus aureus and the highest of 24,19% in Salmonella typhimurium. The results are coincident with those obtained in similar conditions in the Zoo-Prophylactic Venice Institute and others (1, 8, 17).
The growth inhibition effect of pathogen bacteria and the less reduction of bacteria pathogens can be associated to the damage provoked by the action of the Lactoperoxidase system final products on the bacteria structure and/or function, which restrains its restitution even after the effect has disappeared, and then the exacerbation effect is discarded.
Table 3. Reduction Percentage (Activated vs control) of microorganism amount (log10) inoculated in raw milk1./ Porcentaje de reducción (activado vs control) de la cantidad de microorganismos patógenos (log10) inoculados en leche cruda.
 1
1Contamination with 104 ufc/ml for each microorganism, except for Sta. aureos whith 10
6 ufc/ml
5. Effect on Milk Composition and Dairy Products
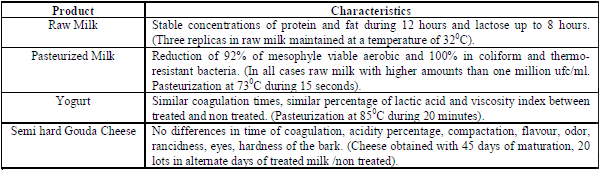
The protein and fat concentrations were stables until 12 hours post activation and until 8 hours in the case of lactose, while activation in highly contaminated milk before the pasteurization process, totally eliminated the presence of coliforms group and thermo resistant bacteria, and did not altered the quality indicators of yogurt, neither the matured cheeses obtained from milk previously activated and pasteurized (Table 4), demonstrating that the method does not change the dairy products quality, including those using lactofermentant bacteria, after an adequate pasteurization of the milk at 850C during 20 minutes.
From the practical point of view, the observed increase on the efficacy of pasteurization is very important in those cases in which the processing milk contains a high bacterial amount, and when failures in the itself process exist, since the total elimination of microorganisms is assured with emphasis in the coliform and pathogen groups. The reduction or elimination of the thermoresistant bacteria vegetative forms and potentially of the spores, is an aspect for further research, but it can be a highly promissory field for long live products and high temperature thermal processes of dairy industry.
The results agree with other reports (17, 18) referring an increase on the efficacy of the thermal process in previously activated milk and the inactivation of the components post activation. The non alteration of cheese and yogurt quality, where live microorganisms are used after the activation and thermal treatment of milk, demonstrates that the LPS is deactivated by the thermal treatment (12, 18, 19). The majority of reports indicating any inhibitor effect on lactofermentant microorganisms have been obtained in none thermally treated milk or with temperatures lower than those established for such processes (85oC during 20 minutes or more).
Table 4. Effect of the LP system activation on the milk components and product quality1 / Efecto de la activación del sLp sobre los componentes lácteos y calidad de los productos1
 1
1In all cases no significant differences or to favor treated milk
6. The Use of the LPs as part of the Integral Programme for Increasing Milk Quality
The inclusion of the Lactoperoxidase system as a method for maintaining the initial quality of raw milk without refrigeration into a program for improving milk quality, included actions related to the milking hygiene and management, mastitis reduction, improvement of the milk solid content, registers and laboratory technical support (11). The integration of a hygienic milking and clean packages is aimed to obtain a good initial quality, meanwhile the use of the LPS activator joined with adequate milk manipulation practices, guarantee the non-deterioration in time and the arrival to optimal quality to industry. A reduction of 90 percent of acid milk arriving to industry and recollection points was obtained and also a decrease in 2 log10 of milk contamination.
The use of the method as part of an integral program for increasing the milk quality and not as an independent measure, assures that the dairy producers use the good practices of milking hygiene and milk manipulation as an essential element of the improvement and the LPs activation as an auxiliary measure to maintain its initial quality (11). On the other hand, though the method improves the pasteurized milk quality indicators, it does not exclude the need of its thermal treatment.
7. New Goals of the Potential Use the Lactoperoxidase System
The LP system activation effects on the saprophytic flora and some pathogen bacteria have been used for other goals besides the milk preservation. It includes the following potential uses.
- Oxidative stress in relation to intestinal inflammation (20,21)
- Treatment without Helicobacter pylori (22, 23, 24)
- Treatment respiratory diseases (25, 26)
- Cosmetics (27)
- Conservation of fish, meats and other foods (4,28,29)
- Conservation of fruits and fruit juices(11, 30, 31)
- Conservation of vegetables from group IV. Substitution of active chloride as disinfectant. (3, 30)
- Use in the development of drugs and dental pastes (23, 32, 33)
- Diagnostic of mastitis in goats (16)
The development of some researches in that field, only used before on milk conservation, shows the high potential of the new uses of the LPs.
Conclusions
Concerning the use of the LP system in Cuba and in other tropical countries from America, the determination of normal thiocyanate ion concentrations and overdosages, together with the confirmation that there is not exacerbation of pathogen bacterias reinforce the criterion of security and innocuity when using the LP system. The calculation of the kinetic of activation as for the combined bacteriostatic and bactericide effects, clarifies the evolution of the activity in time and their scope. The improvement of the efficiency of pasteurization process in milk with high bacterial counting and the experience of activation in milk from four mammal species of economic interest, reinforce the criterion of its use in practice.
There is a coincidence with the conclusions of FAO/ OMS Technical Committee (1), that there are no scientific or practical reasons to maintain the clause that restricts the use of the method in the international milk products market of milk products, which allowed the lifting of the restriction that the system could not be used for intended for international dairy market. The evidence points out to a quick use of the LP system activation for the conservation of raw milk, for other foods and in the development of products for human health.
This article was originally published in Revista de Salud Animal. Vol. 32 No. 3 (2010): 146-154. Reproduced by Scielo. This is an Open Access article licensed under a Creative Commons Attribution License. References
1. FAO/OMS. Benefits and potential risks of the Lps of raw milk preservation. Inform of the technical meeting FAO/OMS. (Monograph Series FAO, 56 pages, 2006 and on the Internet.). Available from: http://www.fao.org/ag/dairy.html.
2. Codex Alimentarius. Thirty-Second Session of the Codex Alimentarius Commission. Alinorm 09/32/ REP, (2009), Rome, Italy.
3. AFSSA. Agencia Francesa de Seguridad de Alimentos. Avis de l´Agence francaise de securité sanitaire des aliments relatif a l´autorisation d¨un systeme lactoperoxidase comme auxilliare technoloque pour traitement des salades Iveme gamme. Saisine Nro 2003-SA-0015.
4. AESA. Agencia Española de Seguridad Alimentaria. Informe del Comité Científico de AESA sobre la utilización del sistema CATALLIC basado en la activación del sistema LP, para el tratamiento de frutas y hortalizas. Nro de Referencia AESA 2005- 012, (2005). España.
5. Uguz MT, Ozdemir H. Purification of bovine milk Lactoperoxidase and investigation of antibacterial properties at different thiocyanate mediated. Appl Bioch Microb. 2005;41:349-53.
6. Kishore B, Banerjee K, Marimuthu P, Bhattacharyya P, Chatterjee M. Effect of thiocyanate through milk on thyroid hormone homeostasis in women. Brit J Nutrit. 1997;78: 679-81.
7. Fernández O, Marrero E, Capdevila JZ. Safety considerations on lactoperoxidase system use for milk preservation. Rev Salud Anim. 2005;27:205- 209.
8. Armenteros M, Dalvit V, Leyva P, Ponce P, Alfonso P. Risk analysis of the exacerbation of foodborne pathogens in raw milk activated with Lactoperoxidase system. Rev Salud Anim. 2006; 29:176- 81.
9. Codex Alimentarius. Request for additional information regardin the potential risk in respect of the lactoperoxidase system. Information from Cuba, Canada, The United States and Canada. CX/FH 07/39/2 Add. 1, October 2007 (2007). India.
10.Codex Alimentarius. Guidelines for the preservation of raw milk by use of the Lactoperoxidase system. CAC/GL 13-1991 (1991).
11.Ponce P. Activación del sistema lactoperoxidasa: un nuevo enfoque para la conservación de leche cruda en el trópico americano. Tesis de Doctor en Ciencias. Centro Nacional de Sanidad Agropecuaria; 2007.
12.Ponce P, Capdevila JZ, Armenteros M. Aspectos prácticos y consideraciones sobre peligros microbiológicos y químicos en el uso sistema lactoperoxidasa en el trópico americano. Rev Salud Anim. 2004;25:163-72.
13.Seifu E, Buys EM, Donki EF. Significance of the lactoperoxidase system in the dairy industry and its potential applications: A Review. Food Sci Techn. 2005;16:137-54.
14.Jacob BM, Essy A, Sreekumar B, Haridas M. Thiocyanate: mediated antifungal and antibacterial property of goat milk lactoperoxidase. Life Sci. 2000;66:2433-39.
15.Revol-Jenelles AM, Milliere JB. The Lactoperoxidase system on milk preservation: Its use, antimicrobial activity and effects on milk products. Global Lactoperoxidase Programme (2005). (GLP)/ FAO, Rome.
16.Seifu E, Donkin EF, Buys ME. Potential of lactoperoxidase to diagnose subclinical mastitis in goats. Small Rum Res. 2007;69:154-58.
17.Kamau DN, Doores S, Pruitt K. Enhanced thermal destruction of Listeria monocitogenes and Staphylococcus aureus by the lactoperoxidase system. Appl Env Microb. Sep. 1990; 2711-16.
18.Barret NE, Grandison AS, Lewis J. Contribution of the Lactoperoxidase to the keeping quality pasteurized milk. J Dairy Res. 1999;66:73-80.
19.Trujillo AJ, Pozo PI, Guamis B. Effect of heat treatment on Lactoperoxidase activity in caprine milk. Small Rum Res. 2007;67:243-246.
20.Davies MJ, Hawkins CL, Pattison DI, Res MD. Mammalian home peroxidase: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1199-234.
21.Matsushita A, Son DO, Satsu H, H. Takano, Kawakami H, H Totsuda et al . Inhibitory effect of lactoperoxidase on the secretion of proinflammatory cytokine interleukin-8 in human epithelial Caco-2 cells. Int Dairy J. 2008;18:923-938.
22.Patent Nro US 6149908. 2000. Use of lactoperoxidase, a peroxide donor and thiocyanate for the manufacture of a medicament for treating Helicobacter pylori infection. Nov 21/ 2000.
23.Haukioja A, Ihalin R, Loimaranta V, Lenander M, Tenuevo J. Sensitivity of Helicobacter pylori toa n innate defense mechanism, the Lactoperoxidase system, in buffer and human whole saliva. J Med Microbiol. 2004; 53: 855-60.
24.Zimecki M, Kruzel ML. Milk-derived proteins and peptides of potential therapeutic and nutritive value. J. Exp. Ther. Oncol. 2007; 6: 89-106.
25.Boots JW, Floris R. Lactoperoxidase: From catalytic mechanism to practical applications. A Review. Int. Dairy J. 2006; 16: 1271-1276.
26.Conner GE, Wijkstrom-Frei C, Randell SH, Fernandez VE, Salathe M. The Lactoperoxidase system links anion transport to host defence in cystic fibrosis. FEBS Lett 2007; 581: 271-278.
27.Glanbia Nutririonals. Lactoperoxidase: Antimicrobials cosmetics. Available at htpp://www. glambianutritionals. com. 2007.
28.Patent Nro US 6 447 811 B1. 2002. Pesticide against plan-pathogenic microorganisms. Sep 10, 2002.
29.Elliot RM, Mclay JC, Kennedy MJ, Simmonds RS. Inhibition of foodborne bacteria by the lactoperoxidase system in a beef cube system. Int J Food Microb. 2005; 91:73-81.
30.Touch V, Hayakawa S, Yamada S, Kaneko S. Effects of a lactoperoxidase-thiocyanate-hydrogen peroxide system on Salmonella enteritidis in animal or vegetable foods. Int J Food Microb. 2004; 93: 175-183.
31.Le Nguyen DD, Ducamp MN, Dornier M, Montet D, Loiseau G. Effect of the lactoperoxidase system against three mayor causal agents of disease in mangoes. J Food Prot. 2005;68: 1497-1500.
32.Shin K, Yamauchi K, Teraguchi S, Hayasawa H, Imoto I. Susceptibility of Helicobacter pylori and its urease activity to the peroxidase-hydrogen peroxide-thiocyanate system. J Med Microb. 2002; 51: 231-237.
33.Korhonen H., Pihlanto A. Technological options for the production of health-promoting proteins and peptides derived from milk and calostrum. Curr Pharm Des. 2007 ; 13: 829-843