1. INTRODUCTION
Histidine (Hist) is one of the 10 essential amino acids for fish, and its importance in nutrition expanding the role of this amino acid beyond its function in protein synthesis. It is well recognized that histidine deficiency can impair growth as well as feed conversion in fish (1, 2). Its metabolites, such as carnosine, anserine and NAH (N-acetyl histidine) play an important role in antioxidant capacity, recovery from fatigue and regulation of osmotic pressure in the eye. In addition, histidine is an important amino acid that contributes to the production of erythrocytes and immune cells in the blood and plays a crucial role in enzymatic reactions due to the side chain of imidazole (3-5). Therefore, in view of its versatile role in fish, care in the dietary inclusion of histidine becomes an extra necessity.
Blood meal is considered to be the richest raw material in histidine (4-6% histidine) (6). After the removal of this ingredient in the mid-1990s, due to the potential risk of transmission of Bovine Spongiform Encephalopathy (BSE), a high incidence of cataracts was observed in Atlantic salmon in Europe and South America (7). Salmonidae are visual predators, and fish with severe cataracts are more susceptible to secondary diseases due to decreased food intake, in addition to having less growth performance.
Therefore, the requirement for histidine for growth (8 g kg-1 feed; (6)) does not meet the need to minimize cataracts in diets for Atlantic salmon. According to Remø, Hevrøy (8), the lowest severity of cataracts can be achieved by feeding 13.4 g kg-1 feed. However, those authors also suggest that the need for histidine in the diet to minimize the risk of cataract development is 14.4 g kg-1 feed of histidine.
From a cost perspective, feed is the most important factor in salmon industry. Since feed represents more than 50% of the operating costs of intensive culture, must be given special attention to cost-effective ingredients sources, to keep feed costs more competitive in the face of fluctuations in raw material prices (9). Since histidine can be produced by fermentation, through a technological and ecologically correct process, which guarantees high quality and safe food. Therefore, this study aimed to evaluate the economic viability of the replacement of blood meal with crystalline L-histidine using the Atlantic salmon (Salmo salar) as an experimental model, which is one of the most cultivated species in the world.
2. METHODS
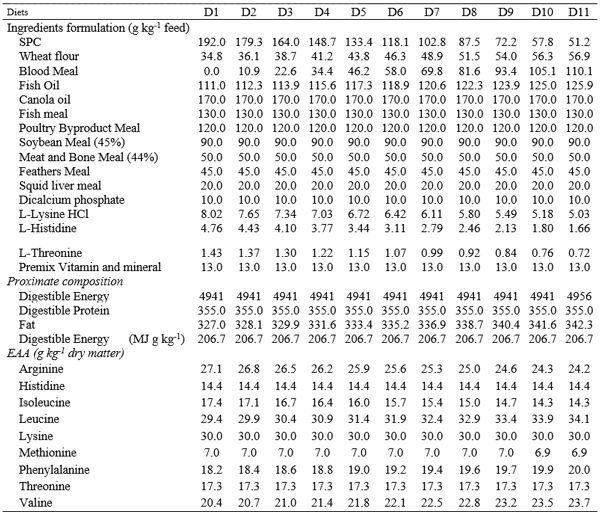
A total of 11 diets were designed to be isoenergetic (20.6 MJ kg-1 DE) and isoproteic/isonitrogenous (40% CP, 35% DP) and to have similar AA profiles. Except for histidine, a balanced simulation diet was formulated to meet the requirement for optimal growth established for Atlantic salmon (Salmon Salar) (10). Histidine level was setup to 1.44% that means that the diets are covering the requirements for the ocular health (8). By adopting that approach based on replacement of BM, protein content and Hist could be meet by using soy protein concentrate (SPC) as source of protein, and also increasing levels of crystalline L-histidine. With the replacement of BM by a vegetable protein source, there was a need for supplementation of crystalline Lys, since this amino acid became limiting in the diet. The inclusion from Canola oil to dicalcium phosphate was kept constant in all diets (Table 1).
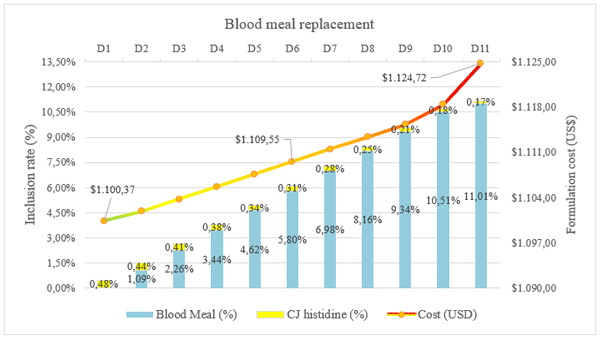
A simulation was conducted to evaluate the economic feasibility of substituting blood meal by crystalline L-histidine in diets for salmon using a minimum cost formulation program. Diets D1 to D11 contained decreasing levels of crystalline L-histidine (0.48, 0.44, 0.41, 0.38, 0.34, 0.31, 0.28, 0.25, 0.21, 0.18, and 0.17 g kg-1 feed).
Table 1. Main ingredients and proximate composition of the diets
3. RESULTS
Result pertaining to economic evaluation of the respective treatments is given in Graphic 1. The economic evaluations were based on the actual feed market prices (11). In all diets, crystalline L-histidine and/or BM contributed at least 32.5% of total dietary histidine. Formulation cost (USD) decreased in response to graded levels of dietary histidine (Graphic 1). The D11 diet had the highest formulation cost reaching US$ 1,124.72, and the difference between the diet D1 was being around US$ 24.35.
Graphic 1. Effect on formulation cost of blood meal replacement by L-histidine in salmon feed
4. DISCUSSION
The supplementation of crystalline L-histidine in feeds for salmon represents an opportunity to reduce formulation costs in the face of traditional commodity market of protein ingredients. An approach to meet the requirements for histidine in Atlantic salmon, while reducing feed costs is achieved through food supplementation with crystalline histidine. Despite the inclusion levels of BM used by the salmon industry not reaching values as high as the maximum proposed in the present study, the total replacement of BM by crystalline L-histidine presented the best formulation cost. With the replacement of BM, there was a need to include soy protein concentrate, since this ingredient is considered a good source of protein-rich ingredient and has the highest apparent digestibility coefficients in salmonids, in addition to the lowest cost (12).
According to Parker (13), the use of animal by-products in feed for farmed Atlantic salmon has a substantial effect on the environmental profile and are associated with most of the impacts of feed production. Animal by-products-free diets can result in substantial improvements, including a 70% reduction in greenhouse gas emissions. Resultantly, its use can fuel an important debate focus into possible means of reducing the aquaculture industry’s dependence on this resource. In addition, such source has often been linked to contaminants, such as pathogens (eg salmonella), which are potentially harmful to fish welfare (14).
L-histidine is produced by an environmentally friendly fermentation technology, through the fermentation of ecological materials. In addition, this amino acid has excellent stability, therefore, its purified crystalline form can be stored in a stable manner at room temperature and also exhibits excellent safety, even in high temperature feed production environments, which is an important point to be considered in the feed salmon production.
Amino acids can cause causing important chemical signals, which stimulate various behavioral reactions, and give an attractive flavor to fish (15). In addition, it is believed that the response of gustatory chemoreceptors in fish is more efficient are fed diets supplemented with crystalline L-histidine (16). Therefore, crystalline L-histidine contributes to increase the attractiveness of diets for animals.
5. CONCLUSION
Results of this study clearly indicate that blood meal could be successfully replaced by L-histidine, and can be considered a correct environmental strategy in salmon nutrition, since it is produced by environmentally friendly fermentation technology. Since blood meal could be associated with impacts on the production of salmon feed, and animal by-products could be associated with contaminants that are potentially harmful to animal welfare, L-histidine can be a key strategy to be safely used in fish feed. The total replacement of blood meal with crystalline L-histidine has substantially improved formulation costs by up to US$ 24.35.







.jpg&w=3840&q=75)