Pigmenting carotenoid sources for salmonid fish
Physiological assessment of natural pigmenting carotenoid sources for salmonid fish: current research and future perspectives
The aquafeed industry in relation to salmonid fish culture is particularly reliant on the use of high quality ingredients such as fish meal and fish oil that constitute the major components of the nutrient-dense feed. However the addition of pigmenting carotenoids is an essential process in order to ensure that the characteristic pink/red flesh colouration is maintained during the production of farm-raised trout and salmon in many countries.
A pink to red colour in the flesh of cultured salmonid fish, especially salmon, is a desirable goal and quite important for consumers with respect to quality and marketing aspects (Skrede and Storebakken, 1986; No and Storebakken, 1991). In salmonid culture this is normally achieved by supplementing fish feeds with carotenoids, typically at concentrations of 40 to 80 mg/ kg (Nickell, 1998). The predominant carotenoid responsible for the coloration of wild salmonids through ingestion of crustaceans is astaxanthin (3, 3'-dihydroxyß, ß-carotene-4, 4'-dione) (Schiedt et al., 1986; Scalia et al., 1989). However, salmonid feeds are commonly supplemented with synthetic astaxanthin and to a much lesser extent the carotenoid canthaxanthin (ß, ß-carotene- 4, 4'-dione) (Bjerkeng, 1992; Bjerkeng et al., 1992; Bell et al., 1998; Akhtar et al., 1999).
Traditional use of commercial synthetic sources of astaxanthin in compounded feeds adds greatly to their costs and the value of the resulting products. The total market for astaxanthin in the salmon industry in 2001 was approximately US$250 million, of which over 90% is primarily the synthetic astaxanthin products of DSM Nutritional Products (Carophyll Pink®) and BASF (Lucantin Pink®) that dominate this important market. The annual sales of synthetic astaxanthin are estimated at more than US$185 million for salmonid fish alone. Recently however, there has been a growing demand in the production of farmed fish under less intensive conditions, and with more emphasis on flesh quality and the use of natural feedstuffs in the diet. This has led to promising research on novel additives and supplements to replace the fish meal protein component, and especially the inclusion of pigmenting agents based on natural astaxanthin sources. A number of investigations have reported the feasibility of single cell products such as the red yeast Phaffia rhodozyma and Haematococcus pluvialis algae with respect to their pigmentation ability compared to the synthetic form currently available to the industry.
Nevertheless, consumer awareness has led to growth in the use of natural sources of feed ingredients (Johnson and An, 1991). Consequently there is much interest in developing new pigment-rich natural sources such as those obtained from algae and yeast products for potential applications in aquafeeds.
Consequently, several commercial products currently available based on this material are being advocated for use in aquafeeds. In Hawaii, Cyanotech Corporation has produced for a number of years a high quality natural astaxanthin rich product, namely (NatuRose®), derived from H. pluvialis. Similarly Igene Biotechnology Inc. produce a high grade Phaffia yeast-based astaxanthin product (AstaXin®) for use in aquaculture as well as other applications with much potential.
In general, some of these natural sources may show variable success mainly due to inconsistencies in the composition and quality of natural organic materials, or the complex biochemical arrangement of the astaxanthin derivatives and the physico-chemical properties associated with their cell wall structure. The prevailing consensus would indicate that the synthetic commercial astaxanthin is readily available for assimilation by fish, and is a more stable and consistent product. Attention has been focused on improving our understanding of carotenoid physiology and biochemistry in salmonid fish such as rainbow trout and salmon with a view towards enhancing the use of astaxanthin as a pigmenting carotenoid and to improve the efficacy of naturally occurring sources through a better understanding of fish utilisation and product composition and processing.
Biotic and abiotic determinants of astaxanthin utilisation in salmonid fish
A number of factors are deemed involved in the rate of astaxanthin deposition in the flesh of fish. These are the rate of growth, as affected by temperature, strain and sex of the animal; water quality criteria, and more importantly the nature of the feed and feed ingredients. With respect to the latter, a number of workers have shown that the level and type of dietary oil is a major factor in the absorption of carotenoids from the intestinal tract of trout and salmon. The protein and energy level of the feed dictates the rate of biomass accumulation (lean tissue mass) in fast growing fish. The rate of muscle fibre recruitment and development is known to influence the binding and hence retention of astaxanthin during the pigmentation phase. There is also an optimum size effect, since only fish above 80 g demonstrate the capacity for carotenoid retention in the flesh. It is unclear as to whether this is related to an inferior gut absorption mechanism that is activated for fish above a size threshold, or hormonal effects. Rainbow trout and salmon are likely to possess the necessary enzyme systems required for the release of astaxanthin from mono- and diesters and various protein complexes found in their natural forms in red yeasts and algae. The free astaxanthin (commercial form) would be expected to be more available under similar dietary inclusion levels, resulting in higher flesh retention values since there would be no prior need for esterase activity. Obviously, a further factor that must be considered is the level of incorporation and the duration of the pigmentation stage in the management of cultured fish or in experimental studies. At present, the acceptable levels range between 25 and 80 ppm in most commercial salmonid diets, and the overall efficiency of net retention is governed by the levels employed for evaluation. For this reason, a target of 50 ppm is normally used in practice for comparative trials.
In recent work undertaken in Plymouth University, the pigmentation effects of a naturally produced astaxanthin product, namely NatuRose® (H. pluvialis) was compared to the main commercial DSM, Carophyll Pink® source at similar dietary levels. Rainbow trout responded favourably, but there was a better efficiency margin for the synthetic source in general for all parameters tested. The degree of esterification at the molecular level was seen to be a major influence on absorption efficiency since the synthetic astaxanthin form currently available is a free astaxanthin and non-esterified in nature. This latter product is suitably coated with a protective proteinstarch envelope to increase stability during storage and processing.
Natural sources of pigmenting carotenoids such as H. pluvialis contain astaxanthin as mono- and diesters that require the pre-hydrolysis at the gastrointestinal lumen interface and uptake within the anterior portion of the intestine in a complex sequence of biochemical and metabolic transformations. The microalga H. pluvialis contains a high amount of astaxanthin (between 1.5- 3.0% dry weight). However, up to 95% of astaxanthin from this source is esterified (~70% monoesters, ~25% diesters; Lorenz and Cysewski, 2000). Following ingestion of astaxanthin esters, intestinal hydrolysis is required before absorption can occur (Schiedt et al., 1986; Storebakken et al., 1987). It has been shown that synthetic astaxanthin di-palmitate is poorly utilised in comparison to free astaxanthin (Foss et al., 1987). Esterification acts to delay uptake and therefore imposes a reduction in the initial rate of absorption of dietary astaxanthin. In addition, the relative feeding rate and frequency of meal presentation to fish is known to affect the transit rate of digesta and resulting digestion and assimilation rate. Body weight, rearing temperature and physiological status also influence metabolism of temperate fish such as trout and salmon. The digestibility and subsequent post- digestive serum astaxanthin levels will relate to these specific processes and could serve as an index of bioavailability and pigmenting efficacy of different astaxanthin sources. All these parameters must be considered in the complete assessment of relative pigmenting capacity as it relates to the visual comparison of fish flesh colour in salmonid fish.
Storebakken et al. (1987) and some authors have reported inferior pigmentation when using feeds supplemented with H. pluvialis compared to supplementation with free astaxanthin (Sommer et al., 1991; 1992). The rate of hydrolysis of astaxanthin esters to free astaxanthin appears to be the limiting factor, and this may explain observed differences in deposition (Torrissen et al., 1989).
Several physiological factors may affect the rate of astaxanthin ester hydrolysis. Firstly, the action of digestive enzymes on nutrients and contact time at absorptive sites in the intestine are affected by transit rate of the feed bolus (Choubert and Storebakken, 1996). Secondly, there may be differences in esterase activity along the length of the salmonid intestine. Finally, there may be differences in carotenoid absorption along the length of the salmonid intestine (Torrissen, 1986; Al-Khalifa and Simpson, 1988) although some authors have reported no variation (Guillou et al., 1992). These physiological constraints have therefore led us to undertake serious investigations on salmonid digestive and gastrointestinal absorption mechanisms. White et al. (2002; 2003) working in Plymouth were amongst the first workers to examine in detail the effects of esterification of astaxanthin from a natural source on absorption physiology in trout. Extracts from these experiments are reported here in detail evaluating NatuRose® Haematococcus and the synthetic DSM freeastaxanthin source Carophyll Pink® at defined dietary levels of 50 mg/kg of feed.
Plasma kinetic studies in trout
Comparisons were made of the ‘steady state’ serum astaxanthin concentrations in rainbow trout, Oncorhynchus mykiss (Walbaum) following daily feeding with esterified (Haematococcus) or free astaxanthin in experimental diets. Secondly, comparisons were made between the absorption rates of astaxanthin in the serum of fish fed a single meal containing each astaxanthin source. The responses between the synthetic astaxanthin and a completely mixed astaxanthin source mainly as esterified astaxanthin shows differences in plasma absorption rates and elimination resulting in a reduced maximum plasma level but slightly longer clearance rate for more complex dietary astaxanthin over a 70 hr assessment period. This is shown by the pattern displayed in Figure 1. In a separate investigation, free astaxanthin and isolated mono and di-esterified astaxanthin were fed at equivalent dietary levels to trout and the subsequent post-prandial kinetic responses measured over 70 hrs. The postprandial profiles of the free astaxanthin, monoester and diester forms are shown in Figure 2. Clearly it is apparent that the absorption of astaxanthin is related to its degree of esterification and that the synthetic free astaxanthin is absorbed faster reaching a higher peak concentration than the monoester with a much lower effect for the diester forms found in natural sources such as H. pluvilialis.
Finally, assessments were made of whether there were any differences along the trout intestine with respect to the hydrolysis of esterified astaxanthin forms. The differential absorption of the various astaxanthin forms was quantified in the experiments conducted by White et al. (2002; 2003) and described later.
A number of natural sources of astaxanthin for fish, including crustacean wastes and krill also contain predominantly esterified astaxanthin. This is often a complex mixture of monoesters and diesters, with unesterified astaxanthin representing a small percentage of the total carotenoid. Astaxanthin is deposited in a free, unesterified form in the white muscle of salmonids, but considerable re-esterification takes place (with fatty acids endogenous to the fish) on deposition in the skin (Schiedt et al., 1986). Subsequently, complete or even partial hydrolysis of these esters within the salmonid gastrointestinal tract prior to absorption may be an important factor limiting uptake and subsequent deposition of natural astaxanthin esters (Choubert and Heinrich, 1993).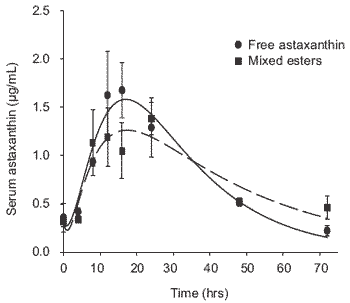
Figure 1. Post-prandial plasma profile of rainbow trout fed free astaxanthin and mixed esters as esterified astaxanthin from extracted H. pluvialis.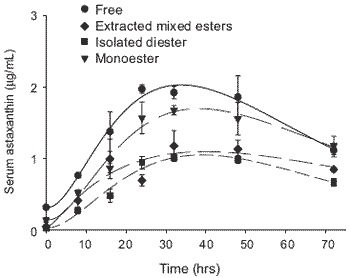
Figure 2. Post-prandial plasma profile of rainbow trout fed free extracted mixed esters (Haematococcus) or as isolated diester and monoester forms of astaxanthin.
The study described by White et al. (2003) has shown that rainbow trout can absorb astaxanthin from either dietary astaxanthin esters or from an unesterified synthetic source to a varying extent with consequences to the efficiency of flesh pigmentation. Blood astaxanthin concentration in these investigations displayed expected high inter-individual variation (Guillou et al., 1992; Gobantes et al., 1997). Attempts to normalise serum astaxanthin concentrations (following ingestion of a single meal) according to the amount of astaxanthin fed to separate groups of trout resulted in no significant reduction in standard errors of the mean values. This may be due to insufficient data concerning individual food consumption. However, other authors have found poor correlations with gut contents and blood astaxanthin concentrations in Atlantic salmon (Kiessling et al., 1995). This may suggest that the amount of feed consumed is only partly responsible for the absorption of astaxanthin.
The mean serum astaxanthin concentrations recorded after 56 days of feeding rainbow trout in the Plymouth trials suggest the absorption of astaxanthin was efficient when fed as a mixture of esters compared to the free form (although mean values were lower). This suggests that intestinal hydrolysis of astaxanthin esters is not limiting under the conditions used in this study. These findings are in agreement with those of Barbosa et al. (1999) who found no significant difference in serum astaxanthin concentrations between rainbow trout fed diets supplemented with either H. pluvialis or synthetic astaxanthin for 5 days. Barbosa et al. (1999) used higher feed carotenoid concentrations (100 ppm) than those in the current study (50 ppm) which may explain why blood astaxanthin concentrations recorded by those authors (ca. 5-9 μg/mL) were higher than reported here.
Analysis of post-prandial serum astaxanthin concentrations after ingestion of a single meal suggests that the absorption rates of astaxanthin are similar when supplied as free or esterified astaxanthin. Similar findings have been noted for Coho salmon (Oncorhynchus kisutch) fed free astaxanthin and an astaxanthin diester derived from krill (Mori et al., 1989). Results would suggest that the rate of absorption of astaxanthin into the blood is not limited by the requirement for hydrolysis when supplied as dietary esters.
These findings are contrary to those of other groups who have demonstrated that synthetic astaxanthin dipalmitate is poorly utilised in comparison to free, unesterified astaxanthin in salmonids (Foss et al., 1987; Storebakken et al., 1987). Since these groups made no direct assessment of absorption of astaxanthin into blood, comparisons with the current study are difficult. However, Storebakken et al. (1987) found no significant differences in mean digestibility values between astaxanthin and astaxanthin dipalmitate. Furthermore, astaxanthin from the esterified source used in the investigation of White was predominantly monoesterified as typical in H. pluvialis. This raises questions concerning the general extent of esterification and its effects on astaxanthin absorption and utilisation in salmonids. However, collaborative work with other groups has demonstrated the effective utilisation of dietary astaxanthin monoesters and diesters for the pigmentation of rainbow trout (Bowen et al., 2001). Experiments support the contention that the anterior/ ileal intestine is largely responsible for carotenoid absorption in fish (Choubert et al., 1987; Al-Khalifa and Simpson, 1988; Torrissen et al., 1990).
Nondetectable levels of astaxanthin mono- and diesters in digesta taken from the ileal and posterior intestine suggest that the hydrolysis of esters prior to absorption also takes place in the pyloric region of the gastrointestinal tract. Regional differences in astaxanthin ester hydrolysis along the length of the trout intestine may result in an influence of gut transit rate on the absorption of astaxanthin from an esterified source.
Furthermore, such effects may account in part for discrepancies between studies on the utilisation of esterified astaxanthin. For example, in those studies by Storebakken et al. (1987) and Foss et al. (1987), which recorded poor utilisation of astaxanthin diester, fish were fed every 20 min for 18 and 24 hrs (to excess) per day, respectively. In comparison, Bowen et al. (2001) fed a restricted ration (1.3-1.8% BW/day) and found no differences in the utilisation of esterified forms of astaxanthin. Although Choubert and Storebakken (1996) found that feeding rate did not affect the digestibility of astaxanthin and canthaxanthin, further research is required on the effects of feeding rate on absorption of esterified carotenoids. Indeed, viable feeding strategies or feed ingredients that extend the gastrointestinal residence time of feed may enhance the absorption of esterified astaxanthin.
Although the encysted wall of H. pluvialis used in the University of Plymouth study was ruptured (95% of cells cracked in a proprietary milling process) it may have posed some further limitation to astaxanthin absorption. Future investigations should be conducted using diets supplemented with astaxanthin fractions isolated from the encysted algal cells. Separate assessment of monoester and diester fractions and the development of parallel in vitro digestion/hydrolysis studies will enhance understanding of carotenoid-ester hydrolysis mechanisms and may prove useful for product development and application.
Gastrointestinal absorption studies
The mechanism of carotenoid absorption in humans and mammals at the gastrointestinal level has been extensively reviewed (Erdman et al., 1993; Parker, 1996; Furr and Clark, 1997; Van den Berg, 1999) yet there is an apparent lack of knowledge relating to similar processes in salmonids. Absorption studies in salmonid species have been assessed through monitoring the elevation and fall of the carotenoid in the blood following meal consumption (Choubert et al., 1994; Kiessling et al., 1995; Gobantes et al., 1997; Aas et al., 1999; White et al., 2002; 2003). However, carotenoid levels in the blood are affected by metabolism and excretion as well as absorption (Castenmiller and West, 1998; Van het Hof et al., 2000). Consequently, this approach, although informative, does not quantify carotenoid absorption. Digestibility studies have been utilised to indicate the intestinal absorption of carotenoid in salmonids (Torrissen et al., 1990; Choubert and Storebakken, 1996; Bjerkeng and Berge, 2000). However, such measurements are often subject to overestimation and variability due to oxidation of carotenoids in faecal samples (Foss et al., 1987; Meyers, 1994). In vitro assessment of intestinal absorption has been conducted successfully for a wide variety of nutrients (Péres del Castillo et al., 1997; Clark et al., 1998). Furthermore, in vitro procedures in comparison to studies of absorption in vivo are favoured by some on both ethical and technical grounds.
Studies have been undertaken at Plymouth and in Norway to evaluate an in vitro everted salmonid intestine model to critically assess the intestinal uptake and provitamin A potential of carotenoids commonly used in salmonid feeds using astaxanthin solubilised in artificial micelle preparations. White et al. (2002) were able to demonstrate that these in vitro experiments produced valuable evidence for the differential uptake of astaxanthin in regions of the gastrointestinal tract. Following incubation in the micelle medium, some carotenoid may have been strongly bound to intestinal mucosal cells despite extensive washing with saline containing bile salts (El-Gorab et al., 1975). Nonabsorbable markers can be utilized in the medium to distinguish between surface-adhered and available substrate (Sallee et al., 1972). However, such methodology often requires the use of radio-labelled markers and solutes so that concurrent analysis of both can take place. Radiolabeled carotenoids are very difficult to obtain and expensive for such investigations.
This finding is supported by other authors who suggest that absorption of carotenoids occurs mainly along the proximal and mid-intestine of salmonids (Torrissen, 1989; White et al., 2002). This is clearly seen in Figure 3 for studies conducted on rainbow trout gut sections in Plymouth. In addition, the absorption of carotenoid by the ileal and hind region of intestine for both Atlantic salmon and rainbow trout is presented in Figure 4, which seems to indicate differences between these species. Variation in nutrient uptake along intestinal regions is not limited to carotenoids and has been also recorded for amino acids in rainbow trout and Coho salmon, Oncorhynchus kisutch (Marcotte and de la Noüe, 1984; Collie, 1985).
Although no statistically significant differences were found for the intestinal absorption of astaxanthin between rainbow trout and Atlantic salmon intestine in these comparative investigations, there is evidence that astaxanthin was better utilized by rainbow trout. March and MacMillan (1996) showed that Atlantic salmon displayed more uniform plasma astaxanthin concentrations regardless of dietary astaxanthin levels, compared to rainbow trout. This led the authors to suggest that the absorptive capacity of the Atlantic salmon intestine limited the amount of astaxanthin that could be advantageously added to their diet. Torrissen et al. (1989), on the basis of reviewed evidence, stated that the digestibility of astaxanthin was greater in rainbow trout (91-97%) compared to Atlantic salmon (45-74%). Indeed other authors have demonstrated interspecies differences between rainbow trout and Atlantic salmon in ability to utilise and efficiently deposit ingested pigment (Foss et al., 1984; Storebakken et al., 1985; Storebakken et al., 1986). The utilization of carotenoids, as determined from final flesh deposition levels, is dependent on an array of physiological and metabolic processes that can individually or indeed mutually have an effect. Blood levels of carotenoids are influenced by physiological processes other than absorption and digestibility values are subject to great variability owing to oxidative degradation of carotenoids in digesta.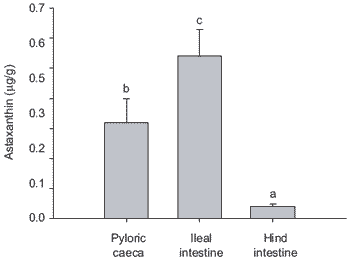
Figure 3. Comparative absorption of astaxanthin by different sections of trout intestine. Bars represent means (n=11, ± SE). Micellar astaxanthin concentration was 5 mg/L. (Bars bearing different letters denote significant differences, P<0.05).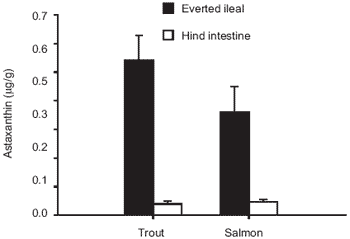
Figure 4. Astaxanthin absorption by everted ileal and hind intestine from both rainbow trout and Atlantic salmon (n=11, ±SE). Astaxanthin concentration in medium was 4.32 ± 0.04 mg/L and 4.30 ± 0.05 mg/L for trout and salmon, respectively (n=3, ± SD).
In the recent study of White et al. (2002), there were clear differences between carotenoids (astaxanthin and canthaxanthin) in terms of the quantities that could be effectively solubilised in artificial bile salt micelles, whereby astaxanthin was incorporated into micelles to a greater extent. This finding is in agreement with other work that has shown differences in micellar incorporation between carotenoids (Tyssandier et al., 1998). Micellar incorporation in vivo is thought to be prerequisite to the intestinal absorption of pigment molecules.
Furthermore, the current data suggest that when astaxanthin and canthaxanthin are combined there is an antagonistic behaviour between these carotenoids in terms of micellar incorporation, i.e. the total incorporation of astaxanthin was significantly reduced. Consequently, combining these carotenoid sources in salmonid feeds may limit their solubility within the gastrointestinal tract, therefore imposing a constraint on utilisation. The interrelationship between these two carotenoids at the level of micelle incorporation merits further study. The gastrointestinal absorption of both astaxanthin and canthaxanthin; as well combinations of both carotenoids are presented in Figure 5. Here, it is evident that there are significant differences in the efficacy of astaxanthin and canthaxanthin absorption by the gastrointestinal tract of rainbow trout. This reflects differences in the retention rates of these carotenoids in practical trials where it is established that trout can better utilize astaxanthin compared to canthaxanthin whilst in salmon the opposite seems to be the case.
Other investigations have shown that rainbow trout utilise dietary astaxanthin to a greater extent when compared to canthaxanthin (Choubert and Storebakken, 1989; Bjerkeng et al., 1990; Storebakken and Choubert, 1991; No and Storebakken, 1992). This additional discrepancy between the two carotenoids may be due to differences in intestinal absorption as indicated by digestibility coefficients (Choubert and Storebakken, 1996); concentrations of the two carotenoids in digestive tract segments (Torrissen, 1986; 1989) and levels in the blood (Guillou et al., 1992; Choubert et al., 1994; Gobantes et al., 1997). Conversely, recent evidence suggests that Atlantic salmon utilise canthaxanthin more efficiently than astaxanthin from feed (Buttle et al., 2001).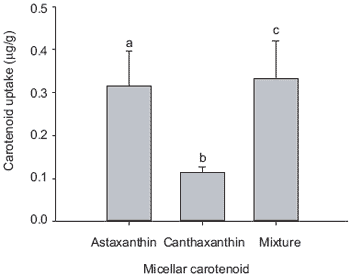
Figure 5. Absorption of astaxanthin (5.40 ± 0.10 mg/L), canthaxanthin (5.10 ± 0.20 mg/L) and a combination of both carotenoids (5.10 ± 0.50 mg/L; n=3 ± SD) by pyloric caeca from rainbow tout (n=11, ± SE). Individual caeca were taken randomly from the pyloric regions of six fish. Values in parenthesies are carotenoid concentrations of the micellar medium. (Bars bearing different letters are significantly different, P<0.05).
Post-absorptive metabolism of astaxanthin
The efficiency of carotenoid absorption from the digestive tract is one factor that influences the retention of carotenoids in salmonids. Moreover, the salmonid intestine seems to be a major site of provitamin A conversion of carotenoids (Schiedt et al., 1985; Al- Khalifa and Simpson, 1988).
It should be noted that vitamin A is essential for a number of physiological processes, such as regulation of cell differentiation and proliferation; vision and reproduction. Several metabolic pathways for the conversion of carotenoids to vitamin A have been suggested in fish (Gross et al., 1966; Hata et al., 1973; Barua and Goswami, 1977; Del Tito, 1983; Schiedt et al., 1985; 1986; Davies and Davies, 1986; Al-Khalifa and Simpson, 1988; Katsuyama and Matsuno, 1988; Guillou et al., 1989; Matsuno, 1991; Yamashita et al., 1996). Evidence from the research investigations in Plymouth by White et al. (2002) suggests that a major fraction of absorbed astaxanthin is transformed into vitamin A in the everted intestinal tissue taken from rainbow trout (Figure 6).
A series of experiments also indicated that there was a significant conversion of astaxanthin into vitamin A in the intestines of rainbow trout previously not exposed to dietary carotenoids. Whether this high conversion ratio has any significance in vivo is difficult to establish since the astaxanthin concentrations used were beyond physiological concentration ranges and the incubation temperature used (20°C) may have influenced the rate of the enzymatic conversion (Guillou et al., 1989).
Nevertheless, provitamin A conversion in the same order of magnitude was found in vivo by Al-Khalifa and Simpson (1988) in intestinal tissue of rainbow trout fed a vitamin A deficient diet for 31 days and fed a single oral dose of 800 μg astaxanthin. The metabolic pathway from astaxanthin to vitamin A has not been elucidated in fish although many pathways have been suggested (see references above). Vitamin A2 is formed either by oxidative conversion of vitamin A1 (Lambertsen and Brækkan, 1969; Schiedt et al., 1985) or by direct conversion from astaxanthin (Katsuyama and Matsuno, 1988; Guillou et al., 1989; Yamashita et al., 1996). An increase in vitamin A caused by conversion of astaxanthin into vitamin A2 should therefore bring about a decrease in the A1:A2 ratio, as was observed in the present study.
To the author’s knowledge, the work in our laboratory in Plymouth is the first in vitro study that demonstrates significant differences in intestinal absorption between the two major carotenoids astaxanthin and canthaxanthin for rainbow trout. The reason for this differential response remains unclear and requires further study to establish the potential selective carotenoid absorption mechanisms and/or variations in transfer of carotenoids between micelles and intestinal tissue.
The evidence for direct conversion of astaxanthin into both vitamin A1 and A2 via selected pathways has important implications relating to the status of this vitamin and associated functions in farmed fish such as salmonids. The question as to what extent astaxanthin is converted into vitamin A and its potential as a provitamin A source in the diet remains to be confirmed by further experimentation. Consequently, the intestinal absorption and metabolism of these carotenoids in the context of improving pigmentation practices whilst promoting fish health and production merits considerable interest.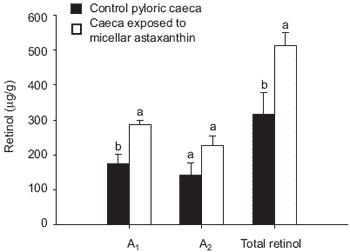
Figure 6. Retinol concentrations (A1, A2 or total retinol) in both control pyloric caeca and caeca exposed to micellar astaxanthin (5.0 ± 0.1mg/L). Bars represent means (n=8, ± SE) (Bars within a grouping bearing different letters are significantly different, P<0.05).
It is apparent that the absorption of dietary carotenoid sources for salmonids is subject to complex processes involving differential uptake mechanisms within the intestine and subject to a post-absorptive metabolism after entering the systemic circulation. The role of the blood and liver in astaxanthin and canthaxanthin metabolism remains to be fully explored. Indeed, Page and Davies (2003) recently conducted isolated perfused liver experiments to elaborate the role of the salmonid liver in carotenoid metabolism with emphasis on astaxanthin and canthaxanthin. Page and Davies (2002) previously examined the uptake and metabolism of these carotenoids in trout hepatocyte tissue culture in order to determine the biochemical enzymatic mechanisms of carotenoid metabolism and quantify losses due to possible conjugation processes associated with the normal hepatic function in these species.
Further studies and strategic research
It is essential that extensive investigations with salmonid fish are conducted in order to better understand the differences between the efficacy of either synthetic carotenoids or natural sources in providing a sound pigmentation program for both salmon and trout. There is much further work to be conducted in improving the processing technology of unicellular carotenoid sources such as Haematococcus and more specifically Phaffia, which contains only free astaxanthin.
The research data presented here indicate considerable scope for refining the composition of natural products containing complex mixtures of astaxanthin esters and opens the question of determining the precise role of astaxanthin in the production of vitamin A and other essential biological functions in fish. Astaxanthin is a potent antioxidant under in vitro conditions and is recognised to be more active that vitamin E. It should also be noted that there is much speculation regarding the exact mechanism for the binding of dietary carotenoids within the accretion of muscle tissue of salmon and trout during growth. It is suggested that astaxanthin may be susceptible to oxidation problems when the antioxidant status of muscle is low or when fish are challenged by various stressors during critical phases of production. There has been some reliable evidence of the synergistic effects of vitamins C and E and more recently selenium (Se) in protecting fish from the effects of free radical damage due to oxidative stress. Supplementation of the diet with these nutrients may result in elevated tissue astaxanthin levels as well as augmenting an increased uniformity of pigment distribution within the muscle structure of harvestable sized salmon and trout.
Beneficial applications in aquaculture could emerge to enhance the utilisation of natural astaxanthin and synthetic forms due to refinement of dietary composition and optimised feeding management. Information leading towards the improvement of carotenoid utilisation efficiency would be of enormous marketable value and commercial interest globally.
Acknowledgments
The author is very appreciative of the work of his former PhD students Drs. Greg Page and Dan White for their novel and peer reviewed work included in this report. The collaboration of the carotenoid research group at John Moores University, Liverpool is fully acknowledged. I thank the support of EWOS, DSM, Cyanotech Corporation and the FAIR grant from the EU, which funded our work in Plymouth. Finally, Robert Serwata was instrumental as always for his role and commitment to our fish nutrition research.
References
Author: SIMON J. DAVIESAas, G.H., B. Bjerkeng, T. Storebakken and B. Ruyter. 1999. Blood appearance, metabolic transformation and plasma transport proteins of 14C-astaxanthin in Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 21:325-334.
Akhtar, P., J.L. Gray, T.H. Cooper, D.L. Garling and A.M. Booren. 1999. Dietary pigmentation and deposition of α-tocopherol and carotenoids in rainbow trout muscle and liver tissue. J. Food Sci. 64:234- 239.
Al-Khalifa, A.S. and K.L. Simpson. 1988. Metabolism of astaxanthin in the rainbow trout (Salmo gairdneri). Comp. Biochem. Physiol. 91B:563-568.
Barbosa, M.J., R. Morais and G. Choubert. 1999. Effect of carotenoid source and dietary lipid content on blood astaxanthin concentration in rainbow trout (Oncorhynchus mykiss). Aquaculture 176:331-341.
Barua, A.B. and U.C. Goswami. 1977. Formation of vitamin A in a freshwater fish - isolation of retinoic acid. J. Biochem. 164:133-136.
Bell, J.G., J. McEvoy, J.L. Webster, F. McGhee, R.M. Millar and J.R. Sargent. 1998. Flesh lipid and carotenoid composition of Scottish farmed Atlantic salmon (Salmo salar). J. Agric. Food Chem. 46:119- 127.
Bjerkeng, B. 1992. Analysis of carotenoids in salmonids. In: Quality Assurance in the Fish Industry (H.H. Huss, M. Jakobsen and J. Liston, eds). Elsevier Science Publishers B.V., pp. 417-424.
Bjerkeng, B. and G.M. Berge. 2000. Apparent digestibility coefficients and accumulation of astaxanthin E/Z isomers in Atlantic salmon (Salmo salar L.) and Atlantic halibut (Hippoglossus hippoglossus L.). Comp. Biochem. Physiol. 127:423- 432.
Bjerkeng, B., T. Storebakken and S. Liaaen-Jensen. 1990. Response to carotenoids by rainbow trout in the sea. Resorption and metabolism of dietary astaxanthin and canthaxanthin. Aquaculture 91:153- 162.
Bjerkeng, B., T. Storebakken and S. Liaaen-Jensen. 1992. Pigmentation of rainbow trout from start feeding to sexual maturation. Aquaculture 108:333-346.
Bowen, J. C. Soutar, R.D. Serwata, S. Lagocki, D.A. White, S.J. Davies and A.J. Young. 2001. Utilisation of (3S,3'S)-astaxanthin acyl esters in pigmentation of rainbow trout (Oncorhynchus mykiss). Aqua. Nutr. 8:59-68.
Buttle, L.G., V.O. Crampton and P.D. Williams. 2001. The effect of feed pigment type on flesh pigment deposition and colour in farmed Atlantic salmon, Salmo salar L. Aqua. Res. 32:103-111.
Castenmiller, J.J.M. and C.E. West. 1998. Bioavailability and bioconversion of carotenoids. Ann. Rev. Nutr. 18:19-38.
Choubert, G. and O. Heinrich. 1993. Carotenoid pigments of the green alga Haematococcus pluvialis: assay on rainbow trout, Oncorhynchus mykiss, pigmentation in comparison with synthetic astaxanthin and canthaxanthin. Aquaculture 112:217-226.
Choubert, G. and T. Storebakken. 1989. Dose response to astaxanthin and canthaxanthin pigmentation of rainbow trout fed various dietary carotenoid concentrations. Aquaculture 81:69-77.
Choubert, G. and T. Storebakken. 1996. Digestibility of astaxanthin and canthaxanthin in rainbow trout as affected by dietary concentration, feeding rate and water salinity. Ann. Zootech. 45:445-453.
Choubert, G., R. Gomez and J.G. Milicua. 1994. Response of serum carotenoid levels to dietary astaxanthin and canthaxanthin in immature rainbow trout Oncorhynchus mykiss. Comp. Biochem. Physiol. 109A:1001-1006.
Choubert, G., A. Guillou and B. Fauconneau. 1987. Absorption and fate of labelled canthaxanthin 15, 15'- 3H2 in rainbow trout (Salmo gairdneri Rich.). Comp. Biochem. Physiol. 87A:717-720.
Clark, R.M., L. Yao, L. She and H.C. Furr. 1998. A comparison of lycopene and canthaxanthin absorption: using the rat to study the absorption of non-provitamin A carotenoids. Lipids 33:159-163.
Collie, N.L. 1985. Intestinal nutrient transport in Coho salmon (Oncorhynchus kisutch) and the effect of development, starvation, and seawater adaptation. J. Comp. Physiol. B. 156:163-174.
Davies, B.W. and B.H. Davies. 1986. Retinol and 3, 4- dehydroretinol formation from xanthophylls in the goldfish, Carassius auratus. Biochem. Soc. Trans. 14:952.
Del Tito, B.J. 1983. Role of ß-carotene and lutein in the synthesis of vitamin A in goldfish. Prog. Fish Cult. 45:94-97.
El-Gorab, M., B.A. Underwood and J.D. Loerch. 1975. The roles of bile salts in the uptake of ß-carotene and retinol by rat everted gut sacs. Biochim. Biophys. Acta. 401:265-277.
Erdman, J.W., T.L. Bierer and E.T. Gugger. 1993. Absorption and transport of carotenoids. Annals. New York Acad. Sci. 691:76-85.
Foss, P., T. Storebakken, E. Austreng and S. Liaaen- Jensen. 1987. Carotenoids in diets for salmonids V. Pigmentation of rainbow trout and sea trout with astaxanthin and astaxanthin dipalmitate in comparison with canthaxanthin. Aquaculture 65:293-305.
Foss, P., T. Storebakken, K. Schiedt, S. Liaaen-Jensen, E. Austreng and K. Streiff. 1984. Carotenoids in diets for salmonids I. Pigmentation of rainbow trout with the individual optical isomers of astaxanthin in comparison with canthaxanthin. Aquaculture 41:213- 226.
Furr, H.C. and R.M. Clark. 1997. Intestinal absorption and tissue distribution of carotenoids. Nutr. Biochem. 8:64-377.
Gobantes, I., G. Choubert, M. Laurentie, J-C.G. Milicua and R. Gomez. 1997. Astaxanthin and canthaxanthin kinetics after ingestion of individual doses by immature rainbow trout Oncorhynchus mykiss. J. Agricult. Food Chem. 45:454-458.
Gross, J. and P. Budowski. 1966. Conversion of carotenoids into vitamin A1 and A2 in two species of freshwater fish. J. Biochem. 101, 747-754.
Guillou, A., G. Choubert and J. de la Nöue. 1992. Absorption and blood clearance of labelled carotenoids ([14C]astaxanthin, [3H]canthaxanthin and [3H]zeaxanthin) in mature female rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. 103A:301-306.
Guillou, A., G. Choubert, T. Storebakken, J. de la Noüe and S. Kaushik. 1989. Bioconversion pathway of astaxanthin into retinol2 in mature rainbow trout (Salmo gairdnieri rich.). Comp. Biochem. Physiol. 94B:481-485.
Guillou, A., G. Choubert and J. de la Nöue. 1992. Comparative accumulations of labelled carotenoids (14C-astaxanthin, 3H-canthaxanthin and 3Hzeaxanthin) and their metabolic conversions in mature female rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. 102B:61-65.
Hata, M., M. Hata and T. Onishi. 1973. Conversion of ß-carotene and retinol1 to retinol2 in freshwater fish. Tohoku J. Agric. Res. 24:197-204.
Johnson, E.A. and G.H. An. 1991. Astaxanthin from microbial sources. Crit. Rev. Biotech. 11:297-326.
Katsuyama, M. and T. Matsuno. 1988. Carotenoid and vitamin A, and metabolism of carotenoids, ß-carotene, canthaxanthin, astaxanthin, zeaxanthin, lutein and tunaxanthin in tilapia Tilapia nilotica. Comp. Biochem. Physiol. 90B:131-139.
Kiessling, A., B. Dosanjh, D. Higgs, G. Deacon and N. Rowshandeli. 1995. Dorsal aorta cannulation: a method to monitor changes in blood levels of astaxanthin in voluntarily feeding Atlantic salmon, Salmo salar L. Aqua. Nutr. 1:43-50.
Kobayashi, M., T. Kakizono and S. Nagai. 1991. Astaxanthin production by a green alga, Haematococcus pluvialis, accompanied with morphological changes in acetate media. J. Ferm. Bioeng. 71:335-339.
Lambertsen, G. and O.R. Brækkan. 1969. In vivo conversion of vitamin A1 to vitamin A2. Act. Chem. Scand. 23:1063-1064.
Lorenz, T.R. and G.R. Cysewski. 2000. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotech. 18:160-167.
March, B.E. and C. MacMillan. 1996. Muscle pigmentation and plasma concentrations of astaxanthin in rainbow trout, chinook salmon, and Atlantic salmon in response to different dietary levels of astaxanthin. Prog. Fish Cult. 58:178-186.
Marcotte, G. and J. de la Nöue. 1984. In vitro intestinal absorption of glycine and L-alanine by rainbow trout, Salmo gairdneri, Rich. Comp. Biochem. Physiol. 79A:209-213.
Matsuno, T. 1991. Xanthophylls as precursors of retinoids. Pure Appl. Chem. 63:81-88.
Meyers, S.P. 1994. Developments in world aquaculture feed formulations, and role of carotenoids. Pure Appl. Chem. 66:1069-1076.
Mori, T., K. Makabe, K. Yamaguchi, S. Konosu and S. Arai. 1989. Comparison between krill astaxanthin diester and synthesized free astaxanthin supplemented to diets in their absorption and deposition by juvenile coho salmon (Oncorhynchus kisutch). Comp. Biochem. Physiol. 93B:255-258.
Nickell, D. 1998. Problems of pigmentation in rainbow trout. Trout News 26:26-30.
No, H.K. and T. Storebakken. 1991. Color stability of rainbow trout fillets during frozen storage. J. Food Sci. 6:969-972, 984.
No, H.K. and T. Storebakken. 1992. Pigmentation of rainbow trout with astaxanthin and canthaxanthin in freshwater and saltwater. Aquaculture 101:123-134.
Page, G. I. and S.J. DaviesJ. 2002. Astaxanthin and canthaxanthin do not induce liver or kidney xenobiotics-metabolising enzymes in rainbow trout, Oncorhynchus mykiss Walbaum. Comp. Biochem. Physiol. (Part C) 133:443-451.
Page, G.I. and S.J. Davies. 2003. Hepatic carotenoid uptake in rainbow trout (Oncorhynchus mykiss) using an isolated organ perfusion model. Aquaculture 225:405-419.
Parker, R.S. 1996. Absorption, metabolism, and transport of carotenoids. FASEB J. 10:542-551.
Perez del Castillo, M.M., M.P. Lostao, A. Barber and F. Ponz. 1997. Some technical precisions to a method for in vivo intestinal absorption studies. J. Physiological Biochemistry 53:281-288.
Sallee, V.L., F.A. Wilson and J.M. Dietschy. 1972. Determination of unidirectional uptake rates for lipids across the intestinal brush border. J. Lipid Res. 13:184- 192.
Scalia, S., M. Isaksen and G.W. Francis. 1989. Carotenoids of the Arctic charr, Salvelinus alpinus (L.). J. Fish Biol. 34:969-970.
Scheidt, K., F.J. Leuenberger, M. Vecchi and E. Glinz. 1985. Absorption, retention and metabolic transformations of carotenoid in rainbow trout, salmon and chicken. Pure Appl. Chem. 57:685-692.
Schiedt, K., M. Vecchi and E. Glinz. 1986. Astaxanthin and its metabolites in wild rainbow trout (Salmo gairdneri R.). Comp. Biochem. Physiol. 83B:9-12.
Skrede, G. and T. Storebakken. 1986. Instrumental colour analysis of farmed and wild Atlantic salmon when raw, baked and smoked. Aquaculture 53:279- 286.
Sommer, T.R., W.T. Potts and N.M. Morrissy. 1991. Utilization of microalgal astaxanthin by rainbow trout (Oncorhynchus mykiss). Aquaculture 94:79-88.
Sommer, T.R., F.M.L. D’Souza and N.M. Morrissy. 1992. Pigmentation of adult rainbow trout, Oncorhynchus mykiss, using the green alga Haematococcus pluvialis. Aquaculture 106:63-74.
Storebakken, T. and G. Choubert. 1991. Flesh pigmentation of rainbow trout fed astaxanthin or canthaxanthin at different feeding rates in freshwater and saltwater. Aquaculture 95:289-295.
Storebakken, T., P. Foss, E. Austreng and S. Liaaen- Jensen. 1985. Carotenoids in diets for salmonids. II. Epimerization studies with astaxanthin in Atlantic salmon. Aquaculture 44:259-269.
Storebakken, T., P. Foss, I. Huse, A. Wandsvik and T.B. Lea. 1986. Carotenoids in diets for salmonids. III. Utilisation of canthaxanthin from dry and wet diets by Atlantic salmon, rainbow trout and sea trout. Aquaculture 51:245-255.
Storebakken, T., P. Foss, K. Schiedt, E. Austreng, S-L. Jensen and U. Manz. 1987. Carotenoids in diets for salmonids IV. Pigmentation of Atlantic salmon with astaxanthin, astaxanthin dipalmitate and canthaxanthin. Aquaculture 65:279-292.
Torrissen, O.J. 1986. Pigmentation of salmonids-a comparison of astaxanthin and canthaxanthin as pigment sources for rainbow trout. Aquaculture 53:271-278.
Torrissen, O.J. 1989. Pigmentation of salmonids: interactions of astaxanthin and canthaxanthin on pigment deposition in rainbow trout. Aquaculture 79:363-374.
Torrissen, O.J., R.W. Hardy and K.D. Shearer. 1989. Pigmentation of salmonids-carotenoid deposition and metabolism. CRC Crit. Rev. Aqua. Sci. 1:209-225.
Torrissen, O.J., R.W. Hardy, K.D. Shearer, T.M. Scott and F.E. Stone. 1990. Effects of dietary canthaxanthin level and lipid level on apparent digestibility coefficients for canthaxanthin in rainbow trout (Oncorhynchus mykiss). Aquaculture 88:351-362.
Tyssandier, V., P. Borel, G. Choubert, P. Grolier, M.C. Alexandre-Gouabau and V. Azais-Braesco. 1998. The bioavailability of carotenoids is positively related to their polarity. Scienza dell’Alimentazione 18:324.
Van den Berg, H. 1999. Carotenoid interactions. Nutr. Rev. 57:1-10.
Van het Hof, K.H., C.E. West, J.A. Weststrate and J.G.A.J. Hautvast. 2000. Dietary factors that affect the bioavailability of carotenoids. J. Nutr. 130:503- 506.
White, D.A., G.I. Page, J. Swaile, J.A. Moody and S.J. Davies. 2002. Effect of esterification on the absorption of astaxanthin in rainbow trout, Oncorhynchus mykiss (Walbaum). Aqua. Res. 33:343-350.
White, D.A., R. Ønsrud and S.J. Davies. 2003. Determination of carotenoid and vitamin A concentrations in everted salmonid intestine following exposure to solutions of carotenoid in vitro. Comp. Biochem. Physiol. (Part A) 136:683-692.
White, D.A., A.J. Moody, R.D. Serwata, J. Bowen, C. Soutar, A.J. Young and S.J. Davies. 2003. The degree of carotenoid esterification influences the absorption of astaxanthin in rainbow trout, Oncorhynchus mykiss (Walbaum). Aqua. Nutr. 9:247-251.
Yamashita, E., S. Arai and T. Matsuno. 1996. Metabolism of xantophylls to vitamin A and new apocarotenoids in liver and skin of black bass, Micropterus salmoides. Comp. Biochem. Physiol. 113B:485-489.
Fish Nutrition Unit, School of Biological Sciences, University of Plymouth, Devon, UK


.jpg&w=3840&q=75)